Position-sensitive scanning fluorescence correlation spectroscopy
- PMID: 15894645
- PMCID: PMC1366613
- DOI: 10.1529/biophysj.105.060749
Position-sensitive scanning fluorescence correlation spectroscopy
Abstract
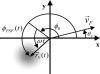
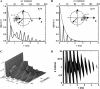
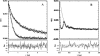
Fluorescence correlation spectroscopy (FCS) uses a stationary laser beam to illuminate a small sample volume and analyze the temporal behavior of the fluorescence fluctuations within the stationary observation volume. In contrast, scanning FCS (SFCS) collects the fluorescence signal from a moving observation volume by scanning the laser beam. The fluctuations now contain both temporal and spatial information about the sample. To access the spatial information we synchronize scanning and data acquisition. Synchronization allows us to evaluate correlations for every position along the scanned trajectory. We use a circular scan trajectory in this study. Because the scan radius is constant, the phase angle is sufficient to characterize the position of the beam. We introduce position-sensitive SFCS (PSFCS), where correlations are calculated as a function of lag time and phase. We present the theory of PSFCS and derive expressions for diffusion, diffusion in the presence of flow, and for immobilization. To test PSFCS we compare experimental data with theory. We determine the direction and speed of a flowing dye solution and the position of an immobilized particle. To demonstrate the feasibility of the technique for applications in living cells we present data of enhanced green fluorescent protein measured in the nucleus of COS cells.
Figures





References
-
- Elson, E. L., and D. Madge. 1974. Fluorescence correlation spectroscopy. I. Conceptual basis and theory. Biopolymers. 12:1–27.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

