Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids
- PMID: 15895105
- PMCID: PMC1576199
- DOI: 10.1038/sj.bjp.0706256
Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids
Abstract
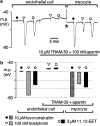
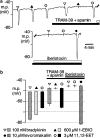
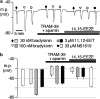
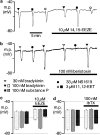
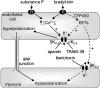
In coronary arteries, bradykinin opens endothelial intermediate- and small-conductance Ca2+-sensitive K+ channels (IK(Ca) and SK(Ca)) and, additionally, releases epoxyeicosatrienoic acids (EETs) from the endothelium. To clarify the involvement of these pathways in endothelium-dependent myocyte hyperpolarization, bradykinin-induced electrical changes in endothelial cells and myocytes of porcine coronary arteries (following nitric oxide (NO) synthase and cyclooxygenase inhibition) were measured using sharp microelectrodes. Hyperpolarization of endothelial cells by bradykinin (27.0 +/- 0.9 mV, n = 4) was partially inhibited (74%) by blockade of IK(Ca) and SK(Ca) channels using 10 microM TRAM-39 (2-(2-chlorophenyl)-2,2-diphenylacetonitrile) plus 100 nM apamin (leaving an iberiotoxin-sensitive component), whereas the response to substance P was abolished. After gap junction blockade with HEPES, (N-(2-hydroxyethyl)piperazine-N'-(2-ethanesulphonic acid)) hyperpolarization of the endothelium by 100 nM bradykinin was abolished by TRAM-39 plus apamin, whereas myocyte hyperpolarization still occurred (12.9 +/- 1.0 mV, n=4). The residual hyperpolarizations to 100 nM bradykinin were antagonized by the EET antagonist, 14,15-EEZE (14,15-epoxyeicosa-5(Z)-enoic acid) (10 microM), and abolished by iberiotoxin. Bradykinin-induced myocyte hyperpolarizations were also reduced by 14,15-EEZE-mSI (14,15-EEZE-methylsulfonylimide) (5,6- and 14,15-EET antagonist), whereas those to exogenous 11,12-EET were unaffected. These data show that bradykinin-induced hyperpolarization of endothelial cells (due to the opening of IK(Ca) and SK(Ca) channels) is electrotonically transferred to the myocytes via gap junctions. Bradykinin (but not substance P) also hyperpolarizes myocytes by a mechanism (independent of endothelial cell hyperpolarization) which involves endothelial cell production of EETs (most likely 14,15- and/or 11,12-EET). These open endothelial IK(Ca) and SK(Ca) channels and also activate large-conductance calcium-sensitive K+ channels (BK(Ca)) on the surrounding myocytes.
Figures



Similar articles
-
Role of gap junctions and EETs in endothelium-dependent hyperpolarization of porcine coronary artery.Br J Pharmacol. 2000 Mar;129(6):1145-54. doi: 10.1038/sj.bjp.0703188. Br J Pharmacol. 2000. PMID: 10725263 Free PMC article.
-
14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries.Circ Res. 2002 May 17;90(9):1028-36. doi: 10.1161/01.res.0000018162.87285.f8. Circ Res. 2002. PMID: 12016270
-
Charybdotoxin-sensitive small conductance K(Ca) channel activated by bradykinin and substance P in endothelial cells.Br J Pharmacol. 2002 Aug;136(8):1201-9. doi: 10.1038/sj.bjp.0704819. Br J Pharmacol. 2002. PMID: 12163354 Free PMC article.
-
Endothelium-derived hyperpolarising factors and associated pathways: a synopsis.Pflugers Arch. 2010 May;459(6):863-79. doi: 10.1007/s00424-010-0817-1. Epub 2010 Apr 11. Pflugers Arch. 2010. PMID: 20383718 Review.
-
Calcium-activated potassium channels and endothelial dysfunction: therapeutic options?Br J Pharmacol. 2009 Feb;156(4):545-62. doi: 10.1111/j.1476-5381.2009.00052.x. Epub 2009 Jan 29. Br J Pharmacol. 2009. PMID: 19187341 Free PMC article. Review.
Cited by
-
Impaired vasodilation in the pathogenesis of hypertension: focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins.J Clin Hypertens (Greenwich). 2012 Apr;14(4):198-205. doi: 10.1111/j.1751-7176.2012.00606.x. J Clin Hypertens (Greenwich). 2012. PMID: 22458740 Free PMC article. Review.
-
Calcium signals that determine vascular resistance.Wiley Interdiscip Rev Syst Biol Med. 2019 Sep;11(5):e1448. doi: 10.1002/wsbm.1448. Epub 2019 Mar 18. Wiley Interdiscip Rev Syst Biol Med. 2019. PMID: 30884210 Free PMC article. Review.
-
Myocardial Ischemia-Reperfusion Injury: Unraveling Pathophysiology, Clinical Manifestations, and Emerging Prevention Strategies.Biomedicines. 2024 Apr 4;12(4):802. doi: 10.3390/biomedicines12040802. Biomedicines. 2024. PMID: 38672157 Free PMC article. Review.
-
Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease.Circulation. 2011 May 24;123(20):2244-53. doi: 10.1161/CIRCULATIONAHA.110.990317. Epub 2011 May 9. Circulation. 2011. PMID: 21555712 Free PMC article. Clinical Trial.
-
Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury: perspectives and implications for postischemic myocardial protection.Am J Transl Res. 2016 Feb 15;8(2):765-77. eCollection 2016. Am J Transl Res. 2016. PMID: 27158368 Free PMC article. Review.
References
-
- ARCHER S.L., GRAGASIN F.S., WU X., WANG S., MCMURTRY S., KIM D.H., PLATONOV M., KOSHAL A., HASHIMOTO K., CAMPBELL W.B., FALCK J.R., MICHELAKIS E.D. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKCa channels. Circulation. 2003;107:769–776. - PubMed
-
- BEVANS C.G., HARRIS A.L. Regulation of connexin channels by pH. Direct action of the protonated form of taurine and other aminosulfonates. J. Biol. Chem. 1999;274:3711––3719. - PubMed
-
- BUSSE R., EDWARDS G., FÉLÉTOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. EDHF: bringing the concepts together. Trends Pharmacol. Sci. 2002;23:374–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

