Key roles of hydrophobic rings of TM2 in gating of the alpha9alpha10 nicotinic cholinergic receptor
- PMID: 15895110
- PMCID: PMC1576203
- DOI: 10.1038/sj.bjp.0706224
Key roles of hydrophobic rings of TM2 in gating of the alpha9alpha10 nicotinic cholinergic receptor
Abstract
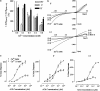
We have performed a systematic mutagenesis of three hydrophobic rings (17', 13' and 9') within transmembrane region (TM) 2 of the alpha9alpha10 nicotinic cholinergic receptor (nAChR) to a hydrophilic (threonine) residue and compared the properties of mutant receptors reconstituted in Xenopus laevis oocytes. Phenotypic changes in alpha9alpha10 mutant receptors were evidenced by a decrease in the desensitization rate, an increase in both the EC(50) for ACh as well as the efficacy of partial agonists and the reduction of the allosteric modulation by extracellular Ca(2+). Mutated receptors exhibited spontaneous openings and, at the single-channel level, an increased apparent mean open time with no major changes in channel conductance, thus suggesting an increase in gating of the channel as the underlying mechanism. Overall, the degrees of the phenotypes of mutant receptors were more overt in the case of the centrally located V13'T mutant. Based on the atomic model of the pore of the electric organ of the Torpedo ray, we can propose that the interactions of side chains at positions 13' and 9' are key ones in creating an energetic barrier to ion permeation. In spite of the fact that the roles of the TM2 residues are mostly conserved in the distant alpha9alpha10 member of the nAChR family, their mechanistic contributions to channel gating show significant differences when compared to other nAChRs. These differences might be originated from slight differential intramolecular rearrangements during gating for the different receptors and might lead each nAChR to be in tune with their physiological roles.
Figures


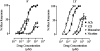


References
-
- AKABAS M.H., KAUFMANN C., ARCHDEACON P., KARLIN A. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the alpha subunit. Neuron. 1994;13:919–927. - PubMed
-
- BERTRAND D., GALZI J.L., DEVILLERS-THIERY A., BERTRAND S., CHANGEUX J.P. Stratification of the channel domain in neurotransmitter receptors. Curr. Opin. Cell Biol. 1993;5:688–693. - PubMed
-
- BERTRAND S., DEVILLERS-THIERY A., PALMA E., BUISSON B., EDELSTEIN S.J., CORRINGER P.J., CHANGEUX J.P., BERTRAND D. Paradoxical allosteric effects of competitive inhibitors on neuronal alpha7 nicotinic receptor mutants. NeuroReport. 1997;8:3591–3596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

