The uncovering of a novel regulatory mechanism for PLD2: formation of a ternary complex with protein tyrosine phosphatase PTP1B and growth factor receptor-bound protein GRB2
- PMID: 15896299
- PMCID: PMC3073396
- DOI: 10.1016/j.bbrc.2005.04.093
The uncovering of a novel regulatory mechanism for PLD2: formation of a ternary complex with protein tyrosine phosphatase PTP1B and growth factor receptor-bound protein GRB2
Abstract
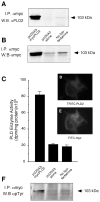
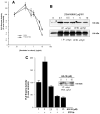
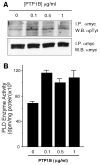
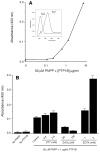
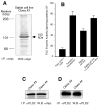
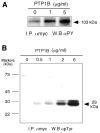
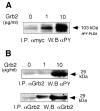
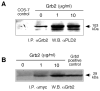
The regulation of PLD2 activation is poorly understood at present. Transient transfection of COS-7 with a mycPLD2 construct results in elevated levels of PLD2 enzymatic activity and tyrosyl phosphorylation. To investigate whether this phosphorylation affects PLD2 enzymatic activity, anti-myc immunoprecipitates were treated with recombinant protein tyrosine phosphatase PTP1B. Surprisingly, lipase activity and PY levels both increased over a range of PTP1B concentrations. These increases occurred in parallel to a measurable PTP1B-associated phosphatase activity. Inhibitor studies demonstrated that an EGF-receptor type kinase is involved in phosphorylation. In a COS-7 cell line created in the laboratory that stably expressed myc-PLD2, PTP1B induced a robust (>6-fold) augmentation of myc-PLD2 phosphotyrosine content. The addition of growth factor receptor-bound protein 2 (Grb2) to cell extracts also elevated PY levels of myc-PLD (>10-fold). Systematic co-immunoprecipitation-immunoblotting experiments pointed at a physical association between PLD2, Grb2, and PTP1B in both physiological conditions and in overexpressed cells. This is the first report of a demonstration of the mammalian isoform PLD2 existing in a ternary complex with a protein tyrosine phosphatase, PTP1b, and the docking protein Grb2 which greatly enhances tyrosyl phosphorylation of the lipase.
Figures
Similar articles
-
New concepts in phospholipase D signaling in inflammation and cancer.ScientificWorldJournal. 2010 Jul 7;10:1356-69. doi: 10.1100/tsw.2010.116. ScientificWorldJournal. 2010. PMID: 20623096 Free PMC article. Review.
-
The exquisite regulation of PLD2 by a wealth of interacting proteins: S6K, Grb2, Sos, WASp and Rac2 (and a surprise discovery: PLD2 is a GEF).Cell Signal. 2011 Dec;23(12):1885-95. doi: 10.1016/j.cellsig.2011.06.017. Epub 2011 Jun 29. Cell Signal. 2011. PMID: 21740967 Free PMC article. Review.
-
The elucidation of novel SH2 binding sites on PLD2.Oncogene. 2006 May 18;25(21):3032-40. doi: 10.1038/sj.onc.1209340. Oncogene. 2006. PMID: 16407827 Free PMC article.
-
The Grb2/PLD2 interaction is essential for lipase activity, intracellular localization and signaling in response to EGF.J Mol Biol. 2007 Mar 30;367(3):814-24. doi: 10.1016/j.jmb.2007.01.021. Epub 2007 Jan 12. J Mol Biol. 2007. PMID: 17276458 Free PMC article.
-
Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein.J Biol Chem. 2000 Feb 11;275(6):4283-9. doi: 10.1074/jbc.275.6.4283. J Biol Chem. 2000. PMID: 10660596
Cited by
-
Structure and regulation of human phospholipase D.Adv Biol Regul. 2021 Jan;79:100783. doi: 10.1016/j.jbior.2020.100783. Epub 2021 Jan 3. Adv Biol Regul. 2021. PMID: 33495125 Free PMC article. Review.
-
New concepts in phospholipase D signaling in inflammation and cancer.ScientificWorldJournal. 2010 Jul 7;10:1356-69. doi: 10.1100/tsw.2010.116. ScientificWorldJournal. 2010. PMID: 20623096 Free PMC article. Review.
-
The molecular basis of phospholipase D2-induced chemotaxis: elucidation of differential pathways in macrophages and fibroblasts.Mol Cell Biol. 2010 Sep;30(18):4492-506. doi: 10.1128/MCB.00229-10. Epub 2010 Jul 20. Mol Cell Biol. 2010. PMID: 20647543 Free PMC article.
-
Short-hairpin RNA-mediated stable silencing of Grb2 impairs cell growth and DNA synthesis.Biochem Biophys Res Commun. 2007 Jun 8;357(3):737-42. doi: 10.1016/j.bbrc.2007.04.013. Epub 2007 Apr 10. Biochem Biophys Res Commun. 2007. PMID: 17445773 Free PMC article.
-
The exquisite regulation of PLD2 by a wealth of interacting proteins: S6K, Grb2, Sos, WASp and Rac2 (and a surprise discovery: PLD2 is a GEF).Cell Signal. 2011 Dec;23(12):1885-95. doi: 10.1016/j.cellsig.2011.06.017. Epub 2011 Jun 29. Cell Signal. 2011. PMID: 21740967 Free PMC article. Review.
References
-
- Klein J, Chalifa V, Liscovitch M, Loffelholz K. Role of phospholipase D activation in nervous system physiology and pathophysiology. J Neurochem. 1995;65:1445–1455. - PubMed
-
- Hammond SM, Altshuller YM, Sung TC, Rudge SA, Rose K, Engebrecht J, Morris AJ, Frohman MA. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J Biol Chem. 1995;270:29640–29643. - PubMed
-
- Hammond SM, Jenco JM, Nakashima S, Cadwallader K, Gu Q, Cook S, Nozawa Y, Prestwich GD, Frohman MA, Morris AJ. Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-alpha. J Biol Chem. 1997;272:3860–3868. - PubMed
-
- Park SK, Provost JJ, Bae CD, Ho WT, Exton JH. Cloning and characterization of phospholipase D from rat brain. J Biol Chem. 1997;272:29263–29271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

