How might cohesin hold sister chromatids together?
- PMID: 15897174
- PMCID: PMC1569479
- DOI: 10.1098/rstb.2004.1604
How might cohesin hold sister chromatids together?
Abstract
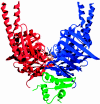
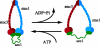
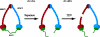
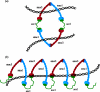
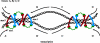
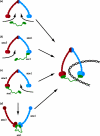
The sister chromatid cohesion essential for the bi-orientation of chromosomes on mitotic spindles depends on a multi-subunit complex called cohesin. This paper reviews the evidence that cohesin is directly responsible for holding sister DNAs together and considers how it might perform this function in the light of recent data on its structure.
Figures

Similar articles
-
Essential roles for cohesin in kinetochore and spindle function in Xenopus egg extracts.J Cell Sci. 2006 Dec 15;119(Pt 24):5057-66. doi: 10.1242/jcs.03277. J Cell Sci. 2006. PMID: 17158911
-
Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1.Nature. 1999 Jul 1;400(6739):37-42. doi: 10.1038/21831. Nature. 1999. PMID: 10403247
-
Cohesin ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation.Nat Cell Biol. 2000 Aug;2(8):492-9. doi: 10.1038/35019529. Nat Cell Biol. 2000. PMID: 10934469
-
Cohesin and DNA damage repair.Exp Cell Res. 2006 Aug 15;312(14):2687-93. doi: 10.1016/j.yexcr.2006.06.024. Epub 2006 Jun 22. Exp Cell Res. 2006. PMID: 16876157 Review.
-
From a single double helix to paired double helices and back.Philos Trans R Soc Lond B Biol Sci. 2004 Jan 29;359(1441):99-108. doi: 10.1098/rstb.2003.1417. Philos Trans R Soc Lond B Biol Sci. 2004. PMID: 15065662 Free PMC article. Review.
Cited by
-
Deterioration without replenishment--the misery of oocyte cohesin.Genes Dev. 2010 Dec 1;24(23):2587-91. doi: 10.1101/gad.2000610. Genes Dev. 2010. PMID: 21123645 Free PMC article.
-
Separase is recruited to mitotic chromosomes to dissolve sister chromatid cohesion in a DNA-dependent manner.Cell. 2009 Apr 3;137(1):123-32. doi: 10.1016/j.cell.2009.01.040. Cell. 2009. PMID: 19345191 Free PMC article.
-
Histone deacetylase 3 is required for centromeric H3K4 deacetylation and sister chromatid cohesion.Genes Dev. 2008 Oct 1;22(19):2639-44. doi: 10.1101/gad.484108. Genes Dev. 2008. PMID: 18832068 Free PMC article.
-
Sex-specific modulation of anxiety-like behavior by forebrain neuronal SMC3 in mice.Transl Psychiatry. 2025 Aug 5;15(1):266. doi: 10.1038/s41398-025-03494-1. Transl Psychiatry. 2025. PMID: 40764477 Free PMC article. Review.
-
Safeguarding entry into mitosis: the antephase checkpoint.Mol Cell Biol. 2010 Jan;30(1):22-32. doi: 10.1128/MCB.00687-09. Mol Cell Biol. 2010. PMID: 19841063 Free PMC article. Review.
References
-
- Arumugan P, et al. ATP hydrolysis is required for cohesin's association with chromosomes. Curr. Biol. 2003;13:1941–1953. - PubMed
-
- Bannister L.A, et al. Positional cloning and characterization of mouse mei8, a disrupted allele of the meiotic cohesin Rec8. Genesis. 2004;40:184–194. - PubMed
-
- Bernard P, et al. Requirement of heterochromatin for cohesion at centromeres. Science. 2001;294:2539–2542. - PubMed
-
- Buonomo S.B, et al. Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell. 2000;103:387–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

