Merotelic kinetochores in mammalian tissue cells
- PMID: 15897180
- PMCID: PMC1569470
- DOI: 10.1098/rstb.2004.1610
Merotelic kinetochores in mammalian tissue cells
Abstract
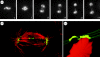
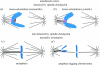
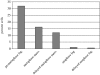
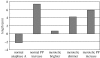
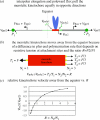
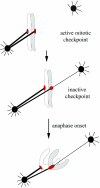
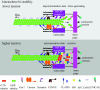
Merotelic kinetochore attachment is a major source of aneuploidy in mammalian tissue cells in culture. Mammalian kinetochores typically have binding sites for about 20-25 kinetochore microtubules. In prometaphase, kinetochores become merotelic if they attach to microtubules from opposite poles rather than to just one pole as normally occurs. Merotelic attachments support chromosome bi-orientation and alignment near the metaphase plate and they are not detected by the mitotic spindle checkpoint. At anaphase onset, sister chromatids separate, but a chromatid with a merotelic kinetochore may not be segregated correctly, and may lag near the spindle equator because of pulling forces toward opposite poles, or move in the direction of the wrong pole. Correction mechanisms are important for preventing segregation errors. There are probably more than 100 times as many PtK1 tissue cells with merotelic kinetochores in early mitosis, and about 16 times as many entering anaphase as the 1% of cells with lagging chromosomes seen in late anaphase. The role of spindle mechanics and potential functions of the Ndc80/Nuf2 protein complex at the kinetochore/microtubule interface is discussed for two correction mechanisms: one that functions before anaphase to reduce the number of kinetochore microtubules to the wrong pole, and one that functions after anaphase onset to move merotelic kinetochores based on the ratio of kinetochore microtubules to the correct versus incorrect pole.
Figures


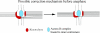

Similar articles
-
Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms.J Cell Sci. 2003 Oct 15;116(Pt 20):4213-25. doi: 10.1242/jcs.00716. Epub 2003 Sep 2. J Cell Sci. 2003. PMID: 12953065
-
Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors.Curr Biol. 2006 Sep 5;16(17):1711-8. doi: 10.1016/j.cub.2006.07.022. Curr Biol. 2006. PMID: 16950108
-
Anaphase spindle mechanics prevent mis-segregation of merotelically oriented chromosomes.Curr Biol. 2004 Dec 14;14(23):2149-55. doi: 10.1016/j.cub.2004.11.029. Curr Biol. 2004. PMID: 15589159
-
Detection and correction of merotelic kinetochore orientation by Aurora B and its partners.Cell Cycle. 2007 Jul 1;6(13):1558-64. doi: 10.4161/cc.6.13.4452. Epub 2007 May 18. Cell Cycle. 2007. PMID: 17603301 Review.
-
Merotelic kinetochore orientation, aneuploidy, and cancer.Biochim Biophys Acta. 2008 Sep;1786(1):32-40. doi: 10.1016/j.bbcan.2008.05.003. Epub 2008 May 23. Biochim Biophys Acta. 2008. PMID: 18549824 Review.
Cited by
-
Hec1/Ndc80 Tail Domain Function at the Kinetochore-Microtubule Interface.Front Cell Dev Biol. 2020 Feb 26;8:43. doi: 10.3389/fcell.2020.00043. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32161753 Free PMC article. Review.
-
Excess centrosomes induce p53-dependent senescence without DNA damage in endothelial cells.FASEB J. 2017 Oct;31(10):4295-4304. doi: 10.1096/fj.201601320R. Epub 2017 Jun 16. FASEB J. 2017. PMID: 28626028 Free PMC article.
-
Semi-automated 3D fluorescence speckle analyzer (3D-Speckler) for microscope calibration and nanoscale measurement.J Cell Biol. 2023 Apr 3;222(4):e202202078. doi: 10.1083/jcb.202202078. Epub 2023 Jan 30. J Cell Biol. 2023. PMID: 36715673 Free PMC article.
-
Aurora B kinase is recruited to multiple discrete kinetochore and centromere regions in human cells.J Cell Biol. 2020 Mar 2;219(3):e201905144. doi: 10.1083/jcb.201905144. J Cell Biol. 2020. PMID: 32028528 Free PMC article.
-
MCAK and paclitaxel have differential effects on spindle microtubule organization and dynamics.Mol Biol Cell. 2009 Mar;20(6):1639-51. doi: 10.1091/mbc.e08-09-0985. Epub 2009 Jan 21. Mol Biol Cell. 2009. PMID: 19158381 Free PMC article.
References
-
- Bharadwaj, R., Qi, W. & Yu., H. T. 2004 Identification of two novel components of the human NDC80 kinetochore complex. J. Biol. Chem.279, 13076–13085. - PubMed
-
- Brinkley, B. R. & Cartwright, Jr., J. 1975 Cold-labile and cold-stable microtubules in the mitotic spindle of mammalian cells. Ann NY Acad. Sci.253, 428–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

