Chromosome bi-orientation on the mitotic spindle
- PMID: 15897181
- PMCID: PMC1569477
- DOI: 10.1098/rstb.2004.1612
Chromosome bi-orientation on the mitotic spindle
Abstract
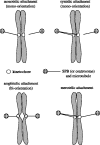
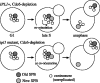
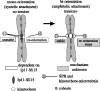
For proper chromosome segregation, sister kinetochores must attach to microtubules extending from opposite spindle poles prior to anaphase onset. This state is called sister kinetochore bi-orientation or chromosome bi-orientation. The mechanism ensuring chromosome bi-orientation lies at the heart of chromosome segregation, but is still poorly understood. Recent evidence suggests that mal-oriented kinetochore-to-pole connections are corrected in a tension-dependent mechanism. The cohesin complex and the Ipl1/Aurora B protein kinase seem to be key regulators for this correction. In this article, I discuss how cells ensure sister kinetochore bi-orientation for all chromosomes, mainly focusing on our recent findings in budding yeast.
Figures
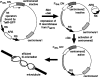
Similar articles
-
Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle.Nature. 2004 Mar 4;428(6978):93-7. doi: 10.1038/nature02328. Epub 2004 Feb 11. Nature. 2004. PMID: 14961024
-
Essential roles for cohesin in kinetochore and spindle function in Xenopus egg extracts.J Cell Sci. 2006 Dec 15;119(Pt 24):5057-66. doi: 10.1242/jcs.03277. J Cell Sci. 2006. PMID: 17158911
-
Mps1 kinase promotes sister-kinetochore bi-orientation by a tension-dependent mechanism.Curr Biol. 2007 Dec 18;17(24):2175-82. doi: 10.1016/j.cub.2007.11.032. Epub 2007 Nov 29. Curr Biol. 2007. PMID: 18060784 Free PMC article.
-
The 'anaphase problem': how to disable the mitotic checkpoint when sisters split.Biochem Soc Trans. 2010 Dec;38(6):1660-6. doi: 10.1042/BST0381660. Biochem Soc Trans. 2010. PMID: 21118144 Review.
-
Bi-orienting chromosomes on the mitotic spindle.Curr Opin Cell Biol. 2002 Jun;14(3):365-71. doi: 10.1016/s0955-0674(02)00328-9. Curr Opin Cell Biol. 2002. PMID: 12067660 Review.
Cited by
-
Aurora B is required for mitotic chromatin-induced phosphorylation of Op18/Stathmin.Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4493-8. doi: 10.1073/pnas.0600702103. Epub 2006 Mar 14. Proc Natl Acad Sci U S A. 2006. PMID: 16537398 Free PMC article.
-
Kinetochore microtubule interaction during S phase in Saccharomyces cerevisiae.Genes Dev. 2007 Dec 15;21(24):3319-30. doi: 10.1101/gad.449407. Genes Dev. 2007. PMID: 18079178 Free PMC article.
-
Phosphorylation of HsMis13 by Aurora B kinase is essential for assembly of functional kinetochore.J Biol Chem. 2008 Sep 26;283(39):26726-36. doi: 10.1074/jbc.M804207200. Epub 2008 Jul 17. J Biol Chem. 2008. PMID: 18640974 Free PMC article.
-
Attaching to spindles before they form: do early incorrect chromosome-microtubule attachments promote meiotic segregation fidelity?Cell Cycle. 2013 Jul 1;12(13):2011-5. doi: 10.4161/cc.25252. Epub 2013 Jun 10. Cell Cycle. 2013. PMID: 23759585 Free PMC article. Review.
-
The chromosome cycle: coordinating replication and segregation. Second in the cycles review series.EMBO Rep. 2005 Nov;6(11):1028-34. doi: 10.1038/sj.embor.7400557. EMBO Rep. 2005. PMID: 16264427 Free PMC article. Review.
References
-
- Adams I.R, Kilmartin J.V. Spindle pole body duplication: a model for centrosome duplication? Trends Cell Biol. 2000;10:329–335. - PubMed
-
- Andrews P.D, Knatko E, Moore W.J, Swedlow J.R. Mitotic mechanics: the auroras come into view. Curr. Opin. Cell Biol. 2003;15:672–683. - PubMed
-
- Andrews P.D, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow J.R. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 2004;6:253–268. - PubMed
-
- Ault J.G, Rieder C.L. Chromosome mal-orientation and reorientation during mitosis. Cell Motil. Cytoskeleton. 1992;22:155–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

