Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells
- PMID: 15897260
- PMCID: PMC2171699
- DOI: 10.1083/jcb.200410081
Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells
Abstract
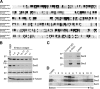
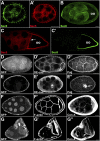
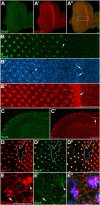
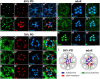
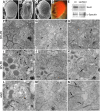
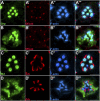
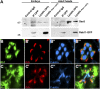
Polarized exocytosis plays a major role in development and cell differentiation but the mechanisms that target exocytosis to specific membrane domains in animal cells are still poorly understood. We characterized Drosophila Sec6, a component of the exocyst complex that is believed to tether secretory vesicles to specific plasma membrane sites. sec6 mutations cause cell lethality and disrupt plasma membrane growth. In developing photoreceptor cells (PRCs), Sec6 but not Sec5 or Sec8 shows accumulation at adherens junctions. In late PRCs, Sec6, Sec5, and Sec8 colocalize at the rhabdomere, the light sensing subdomain of the apical membrane. PRCs with reduced Sec6 function accumulate secretory vesicles and fail to transport proteins to the rhabdomere, but show normal localization of proteins to the apical stalk membrane and the basolateral membrane. Furthermore, we show that Rab11 forms a complex with Sec5 and that Sec5 interacts with Sec6 suggesting that the exocyst is a Rab11 effector that facilitates protein transport to the apical rhabdomere in Drosophila PRCs.
Figures

References
-
- Andrews, H.K., Y.Q. Zhang, N. Trotta, and K. Broadie. 2002. Drosophila sec10 is required for hormone secretion but not general exocytosis or neurotransmission. Traffic. 3:906–921. - PubMed
-
- Ayscough, K.R., J. Stryker, N. Pokala, M. Sanders, P. Crews, and D.G. Drubin. 1997. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137:399–416. - PMC - PubMed
-
- Bucci, C., R.G. Parton, I.H. Mather, H. Stunnenberg, K. Simons, B. Hoflack, and M. Zerial. 1992. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 70:715–728. - PubMed
-
- Chu, D.T., and M.W. Klymkowsky. 1989. The appearance of acetylated alpha-tubulin during early development and cellular differentiation in Xenopus. Dev. Biol. 136:104–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

