Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity
- PMID: 15897263
- PMCID: PMC2171694
- DOI: 10.1083/jcb.200501071
Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity
Abstract
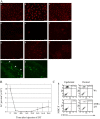
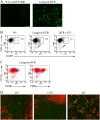
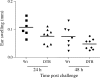
Langerhans cells (LC) form a unique subset of dendritic cells (DC) in the epidermis but so far their in vivo functions in skin immunity and tolerance could not be determined, in particular in relation to dermal DC (dDC). Here, we exploit a novel diphtheria toxin (DT) receptor (DTR)/DT-based system to achieve inducible ablation of LC without affecting the skin environment. Within 24 h after intra-peritoneal injection of DT into Langerin-DTR mice LC are completely depleted from the epidermis and only begin to return 4 wk later. LC deletion occurs by apoptosis in the absence of inflammation and, in particular, the dDC compartment is not affected. In LC-depleted mice contact hypersensitivity (CHS) responses are significantly decreased, although ear swelling still occurs indicating that dDC can mediate CHS when necessary. Our results establish Langerin-DTR mice as a unique tool to study LC function in the steady state and to explore their relative importance compared with dDC in orchestrating skin immunity and tolerance.
Figures


References
-
- Allan, R.S., C.M. Smith, G.T. Belz, A.L. van Lint, L.M. Wakim, W.R. Heath, and F.R. Carbone. 2003. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 301:1925–1928. - PubMed
-
- Anjuere, F., P. Martin, I. Ferrero, M.L. Fraga, G.M. del Hoyo, N. Wright, and C. Ardavin. 1999. Definition of dendritic cell subpopulations present in the spleen, Peyer's patches, lymph nodes, and skin of the mouse. Blood. 93:590–598. - PubMed
-
- Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
-
- Cumberbatch, M., R.J. Dearman, C.E. Griffiths, and I. Kimber. 2003. Epidermal Langerhans cell migration and sensitisation to chemical allergens. APMIS. 111:797–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

