Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response
- PMID: 15897273
- PMCID: PMC2212918
- DOI: 10.1084/jem.20042521
Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response
Abstract
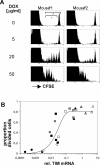
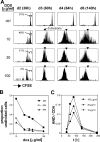
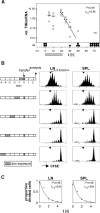
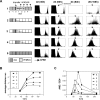
For CD8(+) T cells, a relatively short antigen pulse seems sufficient for antigen-presenting cells to drive clonal expansion and differentiation. It is unknown whether the requirement for antigen is similarly ephemeral for CD4(+) T cells. To study the dependence of a CD4(+) T cell response on antigen persistence in a quantitatively and temporally controlled manner in vivo, we engineered a mouse line expressing a major histocompatibility complex class II-restricted epitope in dendritic cells under the control of a tetracycline-inducible promoter. Experiments tracking the proliferation of CD4(+) T cells exposed to their cognate antigen in various amounts for different time periods revealed that the division of such cells was contingent on the presence of antigen throughout their expansion phase, even in the presence of an inflammatory stimulus. This previously unrecognized feature of a CD4(+) T cell response contrasts with the proliferative behavior of CD8(+) T cells that has been documented, and it implies that the two T cell subsets might require different strategies for efficient vaccination.
Figures


Similar articles
-
Differential kinetics of antigen dependency of CD4+ and CD8+ T cells.J Immunol. 2014 Apr 15;192(8):3507-17. doi: 10.4049/jimmunol.1302725. Epub 2014 Mar 17. J Immunol. 2014. PMID: 24639353
-
Concerted antigen presentation by dendritic cells and B cells is necessary for optimal CD4 T-cell immunity in vivo.Immunology. 2005 Aug;115(4):556-64. doi: 10.1111/j.1365-2567.2005.02196.x. Immunology. 2005. PMID: 16011524 Free PMC article.
-
Stroma-dependent development of two dendritic-like cell types with distinct antigen presenting capability.Exp Hematol. 2013 Mar;41(3):281-92. doi: 10.1016/j.exphem.2012.11.003. Epub 2012 Nov 23. Exp Hematol. 2013. PMID: 23178375
-
CD4+ T cells cooperate with macrophages for specific elimination of MHC class II-negative cancer cells.Adv Exp Med Biol. 2007;590:195-208. doi: 10.1007/978-0-387-34814-8_14. Adv Exp Med Biol. 2007. PMID: 17191387 Review. No abstract available.
-
A role for inflammatory cytokines in the productive activation of antigen-specific CD4+ T-cells.Agents Actions Suppl. 1998;49:23-31. doi: 10.1007/978-3-0348-8857-8_5. Agents Actions Suppl. 1998. PMID: 9426825 Review.
Cited by
-
Azithromycin Clears Bordetella pertussis Infection in Mice but Also Modulates Innate and Adaptive Immune Responses and T Cell Memory.Front Immunol. 2018 Jul 30;9:1764. doi: 10.3389/fimmu.2018.01764. eCollection 2018. Front Immunol. 2018. PMID: 30105030 Free PMC article.
-
Nature and nurture: T-cell receptor-dependent and T-cell receptor-independent differentiation cues in the selection of the memory T-cell pool.Immunology. 2010 Nov;131(3):310-7. doi: 10.1111/j.1365-2567.2010.03338.x. Epub 2010 Aug 25. Immunology. 2010. PMID: 20738422 Free PMC article. Review.
-
Antigen affinity and antigen dose exert distinct influences on CD4 T-cell differentiation.Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14852-7. doi: 10.1073/pnas.1403271111. Epub 2014 Sep 29. Proc Natl Acad Sci U S A. 2014. PMID: 25267612 Free PMC article.
-
BDC12-4.1 T-cell receptor transgenic insulin-specific CD4 T cells are resistant to in vitro differentiation into functional Foxp3+ T regulatory cells.PLoS One. 2014 Nov 13;9(11):e112242. doi: 10.1371/journal.pone.0112242. eCollection 2014. PLoS One. 2014. PMID: 25393309 Free PMC article.
-
Pplase of Dermatophagoides farinae promotes ovalbumin-induced airway allergy by modulating the functions of dendritic cells in a mouse model.Sci Rep. 2017 Feb 27;7:43322. doi: 10.1038/srep43322. Sci Rep. 2017. PMID: 28240301 Free PMC article.
References
-
- Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20:621–667. - PubMed
-
- Itano, A.A., and M.K. Jenkins. 2003. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4:733–739. - PubMed
-
- Catron, D.M., A.A. Itano, K.A. Pape, D.L. Mueller, and M.K. Jenkins. 2004. Visualizing the first 50 hr of the primary immune response to a soluble antigen. Immunity. 21:341–347. - PubMed
-
- Huang, A.Y., H. Qi, and R.N. Germain. 2004. Illuminating the landscape of in vivo immunity: insights from dynamic in situ imaging of secondary lymphoid tissues. Immunity. 21:331–339. - PubMed
-
- Trombetta, S.E., and I. Mellman. 2005. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 23:975–1028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

