Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis
- PMID: 15897276
- PMCID: PMC2212930
- DOI: 10.1084/jem.20050125
Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis
Abstract
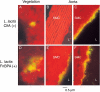
The expression of Staphylococcus aureus adhesins in Lactococcus lactis identified clumping factor A (ClfA) and fibronectin-binding protein A (FnBPA) as critical for valve colonization in rats with experimental endocarditis. This study further analyzed their role in disease evolution. Infected animals were followed for 3 d. ClfA-positive lactococci successfully colonized damaged valves, but were spontaneously eradicated over 48 h. In contrast, FnBPA-positive lactococci progressively increased bacterial titers in vegetations and spleens. At imaging, ClfA-positive lactococci were restricted to the vegetations, whereas FnBPA-positive lactococci also invaded the adjacent endothelium. This reflected the capacity of FnBPA to trigger cell internalization in vitro. Because FnBPA carries both fibrinogen- and fibronectin-binding domains, we tested the role of these functionalities by deleting the fibrinogen-binding domain of FnBPA and supplementing it with the fibrinogen-binding domain of ClfA in cis or in trans. Deletion of the fibrinogen-binding domain of FnBPA did not alter fibronectin binding and cell internalization in vitro. However, it totally abrogated valve infectivity in vivo. This ability was restored in cis by inserting the fibrinogen-binding domain of ClfA into truncated FnBPA, and in trans by coexpressing full-length ClfA and truncated FnBPA on two separate plasmids. Thus, fibrinogen and fibronectin binding could cooperate for S. aureus valve colonization and endothelial invasion in vivo.
Figures





References
-
- Lowy, F.D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532. - PubMed
-
- Foster, T.J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484–488. - PubMed
-
- Arvidson, S. 2000. Extracellular enzymes. Gram-positive Pathogens. V.A. Fischetti, R.P. Novick, J.J. Ferretti, D.A. Portnoy, and J.I. Rood, editors. American Society for Microbiology, Washington, DC. 379–385.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

