Available carbon source influences the resistance of Neisseria meningitidis against complement
- PMID: 15897277
- PMCID: PMC2212924
- DOI: 10.1084/jem.20041548
Available carbon source influences the resistance of Neisseria meningitidis against complement
Abstract
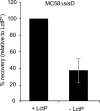
Neisseria meningitidis is an important cause of septicaemia and meningitis. To cause disease, the bacterium must acquire essential nutrients for replication in the systemic circulation, while avoiding exclusion by host innate immunity. Here we show that the utilization of carbon sources by N. meningitidis determines its ability to withstand complement-mediated lysis, through the intimate relationship between metabolism and virulence in the bacterium. The gene encoding the lactate permease, lctP, was identified and disrupted. The lctP mutant had a reduced growth rate in cerebrospinal fluid compared with the wild type, and was attenuated during bloodstream infection through loss of resistance against complement-mediated killing. The link between lactate and complement was demonstrated by the restoration of virulence of the lctP mutant in complement (C3(-/-))-deficient animals. The underlying mechanism for attenuation is mediated through the sialic acid biosynthesis pathway, which is directly connected to central carbon metabolism. The findings highlight the intimate relationship between bacterial physiology and resistance to innate immune killing in the meningococcus.
Figures

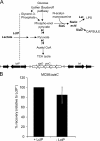
 . (B) Alignment of the predicted amino acid sequence of NMB0543 (accession no. Q9JRD7; EMBL) with LctP from E. coli (accession no. P33231; SwissProt). Identical residues are shown with an asterisk, conserved substitutions are shown with two dots, and semiconserved substitutions are shown with one dot.
. (B) Alignment of the predicted amino acid sequence of NMB0543 (accession no. Q9JRD7; EMBL) with LctP from E. coli (accession no. P33231; SwissProt). Identical residues are shown with an asterisk, conserved substitutions are shown with two dots, and semiconserved substitutions are shown with one dot.


Similar articles
-
HexR Controls Glucose-Responsive Genes and Central Carbon Metabolism in Neisseria meningitidis.J Bacteriol. 2015 Dec 7;198(4):644-54. doi: 10.1128/JB.00659-15. J Bacteriol. 2015. PMID: 26644430 Free PMC article.
-
Antimicrobial peptide resistance in Neisseria meningitidis.Biochim Biophys Acta. 2015 Nov;1848(11 Pt B):3026-31. doi: 10.1016/j.bbamem.2015.05.006. Epub 2015 May 19. Biochim Biophys Acta. 2015. PMID: 26002321 Free PMC article. Review.
-
Neisseria meningitidis lactate permease is required for nasopharyngeal colonization.Infect Immun. 2005 Sep;73(9):5762-6. doi: 10.1128/IAI.73.9.5762-5766.2005. Infect Immun. 2005. PMID: 16113293 Free PMC article.
-
Complement C5a Receptor 1 Exacerbates the Pathophysiology of N. meningitidis Sepsis and Is a Potential Target for Disease Treatment.mBio. 2018 Jan 23;9(1):e01755-17. doi: 10.1128/mBio.01755-17. mBio. 2018. PMID: 29362231 Free PMC article.
-
Molecular mechanisms involved in the interaction of Neisseria meningitidis with cells of the human blood-cerebrospinal fluid barrier.Pathog Dis. 2017 Mar 1;75(2). doi: 10.1093/femspd/ftx023. Pathog Dis. 2017. PMID: 28334198 Review.
Cited by
-
The effects of PspC on complement-mediated immunity to Streptococcus pneumoniae vary with strain background and capsular serotype.Infect Immun. 2010 Jan;78(1):283-92. doi: 10.1128/IAI.00541-09. Epub 2009 Nov 2. Infect Immun. 2010. PMID: 19884335 Free PMC article.
-
Loss of catabolic function in Streptococcus agalactiae strains and its association with neonatal meningitis.J Clin Microbiol. 2006 Sep;44(9):3245-50. doi: 10.1128/JCM.02550-05. J Clin Microbiol. 2006. PMID: 16954255 Free PMC article.
-
Impaired opsonization with C3b and phagocytosis of Streptococcus pneumoniae in sera from subjects with defects in the classical complement pathway.Infect Immun. 2008 Aug;76(8):3761-70. doi: 10.1128/IAI.00291-08. Epub 2008 Jun 9. Infect Immun. 2008. PMID: 18541650 Free PMC article.
-
A long-term field experiment demonstrates the influence of tillage on the bacterial potential to produce soil structure-stabilizing agents such as exopolysaccharides and lipopolysaccharides.Environ Microbiome. 2019 Mar 28;14(1):1. doi: 10.1186/s40793-019-0341-7. Environ Microbiome. 2019. PMID: 33902712 Free PMC article.
-
Immunization with live Neisseria lactamica protects mice against meningococcal challenge and can elicit serum bactericidal antibodies.Infect Immun. 2006 Nov;74(11):6348-55. doi: 10.1128/IAI.01062-06. Epub 2006 Sep 11. Infect Immun. 2006. PMID: 16966413 Free PMC article.
References
-
- Leighton, M.P., D.J. Kelly, M.P. Williamson, and J.G. Shaw. 2001. An NMR and enzyme study of the carbon metabolism of Neisseria meningitidis. Microbiology. 147:1473–1482. - PubMed
-
- Parsons, N.J., G.J. Boons, P.R. Ashton, P.D. Redfern, P. Quirk, Y. Gao, C. Constantinidou, J. Patel, J. Bramley, J.A. Cole, and H. Smith. 1996. Lactic acid is the factor in blood cell extracts which enhances the ability of CMP-NANA to sialylate gonococcal lipopolysaccharide and induce serum resistance. Microb. Pathog. 20:87–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

