Two stable, conducting conformations of the selectivity filter in Shaker K+ channels
- PMID: 15897293
- PMCID: PMC2234082
- DOI: 10.1085/jgp.200509251
Two stable, conducting conformations of the selectivity filter in Shaker K+ channels
Abstract
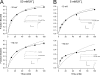
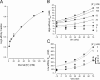
We have examined the voltage dependence of external TEA block of Shaker K(+) channels over a range of internal K(+) concentrations from 2 to 135 mM. We found that the concentration dependence of external TEA block in low internal K(+) solutions could not be described by a single TEA binding affinity. The deviation from a single TEA binding isotherm was increased at more depolarized membrane voltages. The data were well described by a two-component binding scheme representing two, relatively stable populations of conducting channels that differ in their affinity for external TEA. The relative proportion of these two populations was not much affected by membrane voltage but did depend on the internal K(+) concentration. Low internal K(+) promoted an increase in the fraction of channels with a low TEA affinity. The voltage dependence of the apparent high-affinity TEA binding constant depended on the internal K(+) concentration, becoming almost voltage independent in 5 mM. The K(+) sensitivity of these low- and high-affinity TEA states suggests that they may represent one- and two-ion occupancy states of the selectivity filter, consistent with recent crystallographic results from the bacterial KcsA K(+) channel. We therefore analyzed these data in terms of such a model and found a large (almost 14-fold) difference between the intrinsic TEA affinity of the one-ion and two-ion modes. According to this analysis, the single ion in the one-ion mode (at 0 mV) prefers the inner end of the selectivity filter twofold more than the outer end. This distribution does not change with internal K(+). The two ions in the two-ion mode prefer to occupy the inner end of the selectivity filter at low K(+), but high internal K(+) promotes increased occupancy of the outer sites. Our analysis further suggests that the four K(+) sites in the selectivity filter are spaced between 20 and 25% of the membrane electric field.
Figures

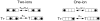
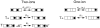


Similar articles
-
External TEA block of shaker K+ channels is coupled to the movement of K+ ions within the selectivity filter.J Gen Physiol. 2003 Aug;122(2):239-46. doi: 10.1085/jgp.200308848. J Gen Physiol. 2003. PMID: 12885878 Free PMC article.
-
Functional identification of ion binding sites at the internal end of the pore in Shaker K+ channels.J Physiol. 2003 May 15;549(Pt 1):107-20. doi: 10.1113/jphysiol.2002.038646. Epub 2003 Mar 28. J Physiol. 2003. PMID: 12665608 Free PMC article.
-
Affinity and location of an internal K+ ion binding site in shaker K channels.J Gen Physiol. 2001 May;117(5):373-84. doi: 10.1085/jgp.117.5.373. J Gen Physiol. 2001. PMID: 11331347 Free PMC article.
-
Ion selectivity in potassium channels.Biophys Chem. 2006 Dec 1;124(3):279-91. doi: 10.1016/j.bpc.2006.05.033. Epub 2006 Jun 18. Biophys Chem. 2006. PMID: 16843584 Review.
-
Permeation energetics in a model potassium channel.Novartis Found Symp. 2002;245:109-22; discussion 122-6, 165-8. Novartis Found Symp. 2002. PMID: 12027003 Review.
Cited by
-
Trans-channel interactions in batrachotoxin-modified skeletal muscle sodium channels: voltage-dependent block by cytoplasmic amines, and the influence of mu-conotoxin GIIIA derivatives and permeant ions.Biophys J. 2008 Nov 1;95(9):4277-88. doi: 10.1529/biophysj.108.138297. Epub 2008 Jul 25. Biophys J. 2008. PMID: 18658222 Free PMC article.
-
Separate gating mechanisms mediate the regulation of K2P potassium channel TASK-2 by intra- and extracellular pH.J Biol Chem. 2010 May 28;285(22):16467-75. doi: 10.1074/jbc.M110.107060. Epub 2010 Mar 29. J Biol Chem. 2010. PMID: 20351106 Free PMC article.
-
Quinidine interaction with Shab K+ channels: pore block and irreversible collapse of the K+ conductance.J Physiol. 2010 Aug 1;588(Pt 15):2691-706. doi: 10.1113/jphysiol.2010.193128. Epub 2010 Jun 14. J Physiol. 2010. PMID: 20547671 Free PMC article.
-
Pharmacology and surface electrostatics of the K channel outer pore vestibule.J Membr Biol. 2006;212(1):51-60. doi: 10.1007/s00232-006-0039-9. Epub 2007 Jan 6. J Membr Biol. 2006. PMID: 17206516 Free PMC article.
-
Ring of negative charge in BK channels facilitates block by intracellular Mg2+ and polyamines through electrostatics.J Gen Physiol. 2006 Aug;128(2):185-202. doi: 10.1085/jgp.200609493. Epub 2006 Jul 17. J Gen Physiol. 2006. PMID: 16847096 Free PMC article.

