Impact of an ectoparasite on the immunity and pathology of an invertebrate: evidence for host immunosuppression and viral amplification
- PMID: 15897457
- PMCID: PMC1140434
- DOI: 10.1073/pnas.0501860102
Impact of an ectoparasite on the immunity and pathology of an invertebrate: evidence for host immunosuppression and viral amplification
Abstract
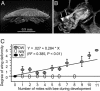
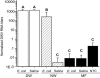
Varroa mites (Varroa destructor) are ectoparasites of honey bees (Apis mellifera) and cause serious damage to bee colonies. The mechanism of how varroa mites kill honey bees remains unclear. We have addressed the effects of the mites on bee immunity and the replication of a picorna-like virus, the deformed wing virus (DWV). The expression of genes encoding three antimicrobial peptides (abaecin, defensin, and hymenoptaecin) and four immunity-related enzymes (phenol oxidase, glucose dehydrogenase, glucose oxidase, and lysozyme) were used as markers to measure the difference in the immune response. We have demonstrated an example of an ectoparasite immunosuppressing its invertebrate host with the evidence that parasitization significantly suppressed expression of these immunity-related genes. Given that ticks immunosuppress their vertebrate hosts, our finding indicates that immunosuppression of hosts may be a common phenomenon in the interaction and coevolution between ectoparasites and their vertebrate and invertebrate hosts. DWV viral titers were significantly negatively correlated with the expression levels of the immunity-related enzymes. All bees had detectable DWV. Mite-infested pupae developed into adults with either normal or deformed wings. All of the deformed-wing bees were greatly infected by DWV (approximately 10(6) times higher than varroa-infested but normal-winged bees). Injection with heat-killed bacteria dramatically promoted DWV titers (10(5) times in 10 h) in the mite-infested, normal-winged bees to levels similar to those found in mite-infested, deformed-wing bees. Varroa mites may cause the serious demise of honey bees by suppressing bee immunity and by boosting the amplification of DWV in bees exposed to microbes.
Figures


References
-
- Schoeler, G. B. & Wikel, S. K. (2001) Ann. Trop. Med. Parasitol. 95, 755–771. - PubMed
-
- Wikel, S. K. & Alarcon-Chaidez, F. J. (2001) Vet. Parasitol. 101, 275–287. - PubMed
-
- Wikel, S. K. (1999) Int. J. Parasitol. 29, 851–859. - PubMed
-
- Waxman, L., Smith, D. E., Arcuri, K. E. & Vlasuk, G. P. (1990) Science 248, 593–596. - PubMed
-
- Titus, R. G. & Ribeiro, J. M. C. (1988) Science 239, 1306–1308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

