Peptide signaling during terminal differentiation of Dictyostelium
- PMID: 15897458
- PMCID: PMC1140433
- DOI: 10.1073/pnas.0501820102
Peptide signaling during terminal differentiation of Dictyostelium
Abstract
A wide variety of mechanisms have evolved for intercellular communication in metazoans, but some of the signaling molecules were already used in their predecessors. The social amoeba, Dictyostelium discoideum, is known to use peptides to trigger sporulation within fruiting bodies, but their sequences have not been defined. We found that the peptide signal spore differentiation factor 2 (SDF-2) is processed from acyl-CoA binding protein, AcbA. The mammalian homolog of AcbA is processed to diazepam binding inhibitor that binds to the GABA(A) receptor in the brain and to peripheral 1,4 benzodiazepine receptors. Although Dictyostelium has neither GABA(A) nor peripheral-type benzodiazepine receptors, we find that both a diazepam binding inhibitor peptide and diazepam (Valium) can mimic SDF-2 in a Dictyostelium bioassay. Mutants lacking AcbA sporulate well only when developed in chimeras with WT cells. Using a yeast system we show that ligand binding to the SDF-2 receptor histidine kinase, DhkA, inhibits phosphorelay, which can account for its ability to induce rapid sporulation.
Figures


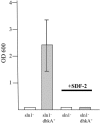

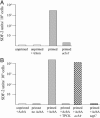

References
-
- Richardson, D. L., Loomis, W. F. & Kimmel, A. R. (1994) Development (Cambridge, U.K.) 120, 2891–2900. - PubMed
-
- Anjard, C., Pinaud, S., Kay, R. R. & Reymond, C. D. (1992) Development (Cambridge, U.K.) 115, 785–790. - PubMed
-
- Anjard, C., Zeng, C. Loomis, W. F. & Nellen, W. (1998) Dev. Biol. 193, 146–155. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

