Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry
- PMID: 15897467
- PMCID: PMC1142358
- DOI: 10.1073/pnas.0409465102
Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry
Abstract
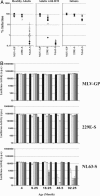
Coronavirus (CoV) infection of humans is usually not associated with severe disease. However, discovery of the severe acute respiratory syndrome (SARS) CoV revealed that highly pathogenic human CoVs (HCoVs) can evolve. The identification and characterization of new HCoVs is, therefore, an important task. Recently, a HCoV termed NL63 was discovered in patients with respiratory tract illness. Here, cell tropism and receptor usage of HCoV-NL63 were analyzed. The NL63 spike (S) protein mediated infection of different target cells compared with the closely related 229E-S protein but facilitated entry into cells known to be permissive to SARS-CoV-S-driven infection. An analysis of receptor engagement revealed that NL63-S binds angiotensin-converting enzyme (ACE) 2, the receptor for SARS-CoV, and HCoV-NL63 uses ACE2 as a receptor for infection of target cells. Potent neutralizing activity directed against NL63- but not 229E-S protein was detected in virtually all sera from patients 8 years of age or older, suggesting that HCoV-NL63 infection of humans is common and usually acquired during childhood. Here, we show that SARS-CoV shares its receptor ACE2 with HCoV-NL63. Because the two viruses differ dramatically in their ability to induce disease, analysis of HCoV-NL63 might unravel pathogenicity factors in SARS-CoV. The frequent HCoV-NL63 infection of humans suggests that highly pathogenic variants have ample opportunity to evolve, underlining the need for vaccines against HCoVs.
Figures
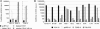
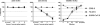
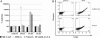
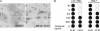
References
-
- Holmes, K. V. (2001) in Fields Virology, eds. Fields, B. N., Knipe, D. M., Howley, P. M., Griffin, D. E., Lamb, R. A., Martin, M. A., Roizman, B. & Straus, S. E. (Lippincott, Williams & Wilkins, Philadelphia), pp. 1187–1203.
-
- Peiris, J. S., Yuen, K. Y., Osterhaus, A. D. & Stohr, K. (2003) N. Engl. J. Med. 349, 2431–2441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

