Foundations of advanced magnetic resonance imaging
- PMID: 15897944
- PMCID: PMC1064985
- DOI: 10.1602/neurorx.2.2.167
Foundations of advanced magnetic resonance imaging
Abstract
During the past decade, major breakthroughs in magnetic resonance imaging (MRI) quality were made by means of quantum leaps in scanner hardware and pulse sequences. Some advanced MRI techniques have truly revolutionized the detection of disease states and MRI can now-within a few minutes-acquire important quantitative information noninvasively from an individual in any plane or volume at comparatively high resolution. This article provides an overview of the most common advanced MRI methods including diffusion MRI, perfusion MRI, functional MRI, and the strengths and weaknesses of MRI at high magnetic field strengths.
Figures






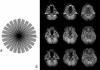
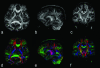
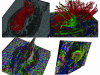
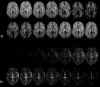


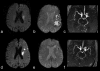

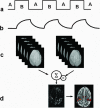
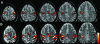

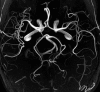



References
-
- Crank J. The mathematics of diffusion. New York: Oxford University Press, 1956.
-
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson 103: 247–254, 1994. - PubMed
-
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8: 333–344, 1995. - PubMed
-
- Lansberg MG, Norbash AM, Marks MP, Tong DC, Moseley ME, Albers GW. Advantages of adding diffusion-weighted magnetic resonance imaging to conventional magnetic resonance imaging for evaluating acute stroke. Arch Neurol 57: 1311–1316, 2000. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

