Imaging of multiple sclerosis: role in neurotherapeutics
- PMID: 15897951
- PMCID: PMC1064992
- DOI: 10.1602/neurorx.2.2.277
Imaging of multiple sclerosis: role in neurotherapeutics
Abstract
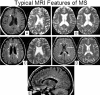
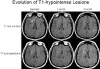
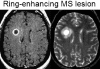
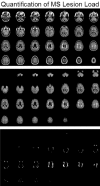
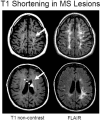
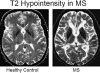
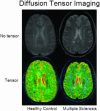
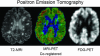
Magnetic resonance imaging (MRI) plays an ever-expanding role in the evaluation of multiple sclerosis (MS). This includes its sensitivity for the diagnosis of the disease and its role in identifying patients at high risk for conversion to MS after a first presentation with selected clinically isolated syndromes. In addition, MRI is a key tool in providing primary therapeutic outcome measures for phase I/II trials and secondary outcome measures in phase III trials. The utility of MRI stems from its sensitivity to longitudinal changes including those in overt lesions and, with advanced MRI techniques, in areas affected by diffuse occult disease (the so-called normal-appearing brain tissue). However, all current MRI methodology suffers from limited specificity for the underlying histopathology. Conventional MRI techniques, including lesion detection and measurement of atrophy from T1- or T2-weighted images, have been the mainstay for monitoring disease activity in clinical trials, in which the use of gadolinium with T1-weighted images adds additional sensitivity and specificity for areas of acute inflammation. Advanced imaging methods including magnetization transfer, fluid attenuated inversion recovery, diffusion, magnetic resonance spectroscopy, functional MRI, and nuclear imaging techniques have added to our understanding of the pathogenesis of MS and may provide methods to monitor therapies more sensitively in the future. However, these advanced methods are limited by their cost, availability, complexity, and lack of validation. In this article, we review the role of conventional and advanced imaging techniques with an emphasis on neurotherapeutics.
Figures
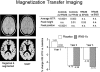
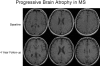
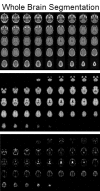
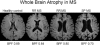
References
-
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 121–127, 2001. - PubMed
-
- Miller DH, Filippi M, Fazekas F, Federiksen JL, Matthews PM, Montalban X, et al. Role of magnetic resonance imaging within diagnostic criteria for multiple sclerosis. Ann Neurol 56: 273–278, 2004. - PubMed
-
- Barkhof F, Rocca M, Francis G, Van Waesberghe JH, Uitdehaag BM, Hommes OR, et al. Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon β1a. Ann Neurol 53: 718–724, 2003. - PubMed
-
- Zivadinov R, Bakshi R. Role of MRI in multiple sclerosis I: inflammation and lesions. Front Biosci 9: 665–683, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

