Imaging in neurooncology
- PMID: 15897954
- PMCID: PMC1064995
- DOI: 10.1602/neurorx.2.2.333
Imaging in neurooncology
Abstract
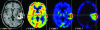
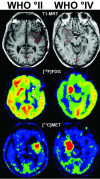
Imaging in patients with brain tumors aims toward the determination of the localization, extend, type, and malignancy of the tumor. Imaging is being used for primary diagnosis, planning of treatment including placement of stereotaxic biopsy, resection, radiation, guided application of experimental therapeutics, and delineation of tumor from functionally important neuronal tissue. After treatment, imaging is being used to quantify the treatment response and the extent of residual tumor. At follow-up, imaging helps to determine tumor progression and to differentiate recurrent tumor growth from treatment-induced tissue changes, such as radiation necrosis. A variety of complementary imaging methods are currently being used to obtain all the information necessary to achieve the above mentioned goals. Computed tomography and magnetic resonance imaging (MRI) reveal mostly anatomical information on the tumor, whereas magnetic resonance spectroscopy and positron emission tomography (PET) give important information on the metabolic state and molecular events within the tumor. Functional MRI and functional PET, in combination with electrophysiological methods like transcranial magnetic stimulation, are being used to delineate functionally important neuronal tissue, which has to be preserved from treatment-induced damage, as well as to gather information on tumor-induced brain plasticity. In addition, optical imaging devices have been implemented in the past few years for the development of new therapeutics, especially in experimental glioma models. In summary, imaging in patients with brain tumors plays a central role in the management of the disease and in the development of improved imaging-guided therapies.
Figures




References
-
- Kleihues P, Soylemezoglu F, Schauble B, Scheithauer BW, Burger PC. Histopathology, classification, and grading of gliomas. Glia 15: 211–221, 1995. - PubMed
-
- Barker FG, Israel MA. Molecular genetics. In: The gliomas (Berger MS, Wilson CB, eds), pp 39–51. Philadelphia: W.B. Saunders Co., 1999.
-
- Ichimura K, Bolin MB, Goike HM, Schmidt EE, Moshref A, Collins VP. Deregulation of the p14ARF/MDM2/p53 pathway is a prerequisite for human astrocytic gliomas with G1-S transition control gene abnormalities. Cancer Res 60: 417–424, 2000. - PubMed
-
- Morrison RS. Growth factor mediated signaling pathways. In: The gliomas (Berger MS, Wilson CB, eds), pp 52–64. Philadelphia: W.B. Saunders Co., 1999.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

