Connexin43, the major gap junction protein of astrocytes, is down-regulated in inflamed white matter in an animal model of multiple sclerosis
- PMID: 15898103
- PMCID: PMC1226319
- DOI: 10.1002/jnr.20474
Connexin43, the major gap junction protein of astrocytes, is down-regulated in inflamed white matter in an animal model of multiple sclerosis
Abstract
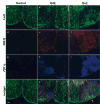
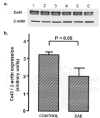
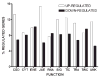
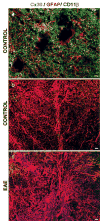
Both multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE), its animal model, involve inflammatory attack on central nervous system (CNS) white matter, leading to demyelination and axonal damage. Changes in astrocytic morphology and function are also prominent features of MS and EAE. Resting astrocytes form a network that is interconnected through gap junctions, composed mainly of connexin43 (Cx43) protein. Although astrocytic gap junctional connectivity is known to be altered in many CNS pathologies, little is known about Cx43 expression in inflammatory demyelinating disease. Therefore, we evaluated the expression of Cx43 in spinal cords of EAE mice compared with healthy controls. Lumbar ventral white matter areas were heavily infiltrated with CD11beta-immunoreactive monocytes, and within these infiltrated regions loss of Cx43 immunoreactivity was evident. These regions also showed axonal dystrophy, demonstrated by the abnormally dephosphorylated heavy-chain neurofilament proteins. Astrocytes in these Cx43-depleted lesions were strongly glial fibrillary acidic protein reactive. Significant loss (38%) of Cx43 protein in EAE mouse at the lumbar portion of spinal cords was confirmed by Western blot analysis. Decreased Cx43 transcript level was also observed on cDNA microarray analysis. In addition to changes in Cx43 expression, numerous other genes were altered, including those encoding adhesion and extracellular matrix proteins. Our data support the notion that, in addition to damage of myelinating glia, altered astrocyte connectivity is a prominent feature of inflammatory demyelination.
Copyright 2005 Wiley-Liss, Inc.
Figures


References
-
- Adams RD, Kubik CS. The morbid anatomy of the demyelinative disease. Am J Med. 1952;12:510–546. - PubMed
-
- Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10:2045–2058. - PubMed
-
- Bauer J, Berkenbosch F, Van Dam AM, Dijkstra CD. Demonstration of interleukin-1 beta in Lewis rat brain during experimental allergic encephalomyelitis by immunocytochemistry at the light and ultra-structural level. J Neuroimmunol. 1993;48:13–21. - PubMed
-
- Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkestra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15:437–446. - PubMed
-
- Benn T, Halfpenny C, Scolding N. Glial cells as targets for cytotoxic immune mediators. Glia. 2001;36:200–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

