Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity
- PMID: 15899032
- PMCID: PMC1174941
- DOI: 10.1186/ar1697
Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity
Abstract
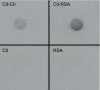
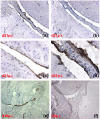
Autoantibodies directed against citrulline-containing proteins have an impressive specificity of nearly 100% in patients with rheumatoid arthritis and have been suggested to be involved in the disease pathogenesis. The targeted epitopes are generated by a post-translational modification catalysed by the calcium-dependent enzyme peptidyl arginine deiminase (PAD), which converts positively charged arginine to polar but uncharged citrulline. The aim of this study was to explore the effects of citrullination on the immunogenicity of autoantigens as well as on potential arthritogenicity. Thus, immune responses to citrullinated rat serum albumin (Cit-RSA) and to unmodified rat serum albumin (RSA) were examined as well as arthritis development induced by immunisation with citrullinated rat collagen type II (Cit-CII) or unmodified CII. In addition, to correlate the presence of citrullinated proteins and the enzyme PAD4 with different stages of arthritis, synovial tissues obtained at different time points from rats with collagen-induced arthritis were examined immunohistochemically. Our results demonstrate that citrullination of the endogenous antigen RSA broke immunological tolerance, as was evident by the generation of antibodies directed against the modified protein and cross-reacting with the native protein. Furthermore we could demonstrate that Cit-CII induced arthritis with higher incidence and earlier onset than did the native counterpart. Finally, this study reveals that clinical signs of arthritis precede the presence of citrullinated proteins and the enzyme PAD4. As disease progressed into a more severe and chronic state, products of citrullination appeared specifically in the joints. Citrullinated proteins were detected mainly in extracellular deposits but could also be found in infiltrating cells and on the cartilage surface. PAD4 was detected in the cytoplasm of infiltrating mononuclear cells, from day 21 after immunisation and onwards. In conclusion, our data reveal the potency of citrullination to break tolerance against the self antigen RSA and to increase the arthritogenic properties of the cartilage antigen CII. We also show that citrullinated proteins and the enzyme PAD4 are not detectable in healthy joints, and that the appearance and amounts in arthritic joints of experimental animals are correlated with the severity of inflammation.
Figures


References
-
- Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, Vincent C, Simon M, Senshu T, Masson-Bessiere C, Jolivet-Reynaud C, et al. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

