Small GTP-binding protein Rho-mediated signaling promotes proliferation of rheumatoid synovial fibroblasts
- PMID: 15899034
- PMCID: PMC1174939
- DOI: 10.1186/ar1694
Small GTP-binding protein Rho-mediated signaling promotes proliferation of rheumatoid synovial fibroblasts
Abstract
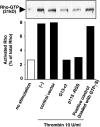
Rho is a major small GTP-binding protein that is involved in the regulation of various cell functions, including proliferation and cell migration, through activation of multiple signaling molecules in various types of cells. We studied its roles in synovial fibroblasts (SFs) in patients with rheumatoid arthritis (RA) and clarified its relevance to RA synovitis, with the following results. 1)We found that the thrombin receptor was overexpressed on RA synovial fibroblasts (RA SFs) and that thrombin induced a marked proliferation and progression of the cell cycle to the S phase in these cells. 2)We also found that thrombin efficiently activated Rho. 3)Rho activation and proliferation and the progression of the cell cycle to the S phase were completely blocked by p115RGS (an N-terminal regulator of the G-protein signaling domain of p115RhoGEF) and by the C-terminal fragments of Galpha13 (an inhibitor of the interaction of receptors with G13). 4)Thrombin induced the secretion of IL-6 by RA SFs, but this action was blocked by p115RGS or Galpha13. Our findings show that the actions of thrombin on the proliferation of RA SFs, cell-cycle progression to the S phase, and IL-6 secretion were mainly mediated by the G13 and RhoGEF pathways. These results suggest that p115RGS and Galpha13 could be potent inhibitors of such functions. A rational design of future therapeutic strategies for RA synovitis could perhaps include the exploitation of the Rho pathway to directly reduce the growth of synovial cells.
Figures




References
-
- Yamamoto M, Marui N, Sakai T, Morii N, Kozaki S, Ikai K, Imamura S, Narumiya S. ADP-ribosylation of the rhoA gene product by botulinum C3 exoenzyme causes Swiss 3T3 cells to accumulate in the G1 phase of the cell cycle. Oncogene. 1993;8:1449–1455. - PubMed
-
- Rodriguez-Fernandez JL, Sanchez-Martin L, Rey M, Vicente-Manzanares M, Narumiya S, Teixido J, Sanchez-Madrid F, Cabanas C. Rho and Rho-associated kinase modulate the tyrosine kinase PYK2 in T-cells through regulation of the activity of the integrin LFA-1. J Biol Chem. 2001;276:40518–40527. doi: 10.1074/jbc.M102896200. - DOI - PubMed
-
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

