Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports
- PMID: 15899051
- PMCID: PMC1174947
- DOI: 10.1186/ar1704
Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports
Erratum in
- Arthritis Res Ther. 2006;8(1):401
Abstract
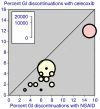
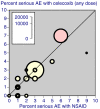
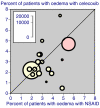
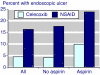
The objective was to improve understanding of adverse events occurring with celecoxib in the treatment of osteoarthritis and rheumatoid arthritis. Data were extracted from company clinical trial reports of randomised trials of celecoxib in osteoarthritis or rheumatoid arthritis lasting 2 weeks or more. Outcomes were discontinuations (all cause, lack of efficacy, adverse event, gastrointestinal adverse event), endoscopically detected ulcers, gastrointestinal or cardio-renal events, and major changes in haematological parameters. The main comparisons were celecoxib (all doses) versus placebo, paracetamol (acetaminophen) 4,000 mg daily, rofecoxib 25 mg daily, or nonsteroidal anti-inflammatory drugs (NSAIDs) (naproxen, diclofenac, ibuprofen, and loxoprofen). For NSAIDs, celecoxib was compared both at all doses and at licensed doses (200 to 400 mg daily). Thirty-one trials included 39,605 randomised patients. Most patients had osteoarthritis and were women of average age 60 years or above. Most trials lasted 12 weeks or more. Doses of celecoxib were 50 to 800 mg/day. Compared with placebo, celecoxib had fewer discontinuations for any cause or for lack of efficacy, fewer serious adverse events, and less nausea. It had more patients with dyspepsia, diarrhoea, oedema, more adverse events that were gastrointestinal or treatment related, and more patients experiencing an adverse event. There were no differences for hypertension, gastrointestinal tolerability, or discontinuations for adverse events. Compared with paracetamol, celecoxib had fewer discontinuations for any cause, for lack of efficacy, or diarrhoea, but no other differences. Compared with rofecoxib, celecoxib had fewer patients with abdominal pain and oedema, but no other differences. Compared with NSAIDs, celecoxib had fewer symptomatic ulcers and bleeds, endoscopically detected ulcers, and discontinuations for adverse events or gastrointestinal adverse events. Fewer patients had any, or a gastrointestinal, or a treatment-related adverse event, or vomiting, abdominal pain, dyspepsia, or reduced haemoglobin or haematocrit. Discontinuations for lack of efficacy were higher. No differences were found for all-cause discontinuations, serious adverse events, hypertension, diarrhoea, nausea, oedema, myocardial infarction, cardiac failure, or raised creatinine. Company clinical trial reports present much more information than published papers. Adverse event information is clearly presented in company clinical trial reports, which are an ideal source of information for systematic review and meta-analysis.
Figures
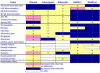
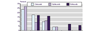
References
-
- Scholes D, Stergachis A, Penna PM, Normand EH, Hansten PD. Nonsteroidal anti-inflammatory drug discontinuation in patients with osteoarthritis. J Rheumatol. 1995;22:708–712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

