Distinct structural domains within C19ORF5 support association with stabilized microtubules and mitochondrial aggregation and genome destruction
- PMID: 15899810
- PMCID: PMC3225222
- DOI: 10.1158/0008-5472.CAN-04-3865
Distinct structural domains within C19ORF5 support association with stabilized microtubules and mitochondrial aggregation and genome destruction
Abstract
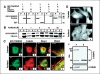
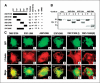
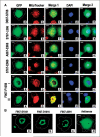
C19ORF5 is a sequence homologue of microtubule-associated proteins MAP1A/MAP1B of unknown function, except for its association with mitochondria-associated proteins and the paclitaxel-like microtubule stabilizer and candidate tumor suppressor RASSF1A. Here, we show that when overexpressed in mammalian cells the recombinant 393-amino acid residue COOH terminus of C19ORF5 (C19ORF5C) exhibited four types of distribution patterns proportional to expression level. Although normally distributed throughout the cytosol without microtubular association, C19ORF5C specifically accumulated on stabilized microtubules in paclitaxel-treated cells and interacted directly with paclitaxel-stabilized microtubules in vitro. The native 113-kDa full-length C19ORF5 and a shorter 56-kDa form similarly associated with stabilized microtubules in liver cells and stabilized microtubules from their lysates. As C19ORF5 accumulated, it appeared on mitochondria and progressively induced distinct perinuclear aggregates of mitochondria. C19ORF5 overlapped with cytochrome c-deficient mitochondria with reduced membrane potential. Mitochondrial aggregation resulted in gross degradation of DNA, a cell death-related process we refer to as mitochondrial aggregation and genome destruction (MAGD). Deletion mutagenesis revealed that the C19ORF5 hyperstabilized microtubule-binding domain resides in a highly basic sequence of <100 residues, whereas the MAGD activity resides further downstream in a distinct 25-residue sequence (F967-A991). Our results suggest that C19ORF5 mediates communication between the microtubular cytoskeleton and mitochondria in control of cell death and defective genome destruction through distinct bifunctional structural domains. The accumulation of C19ORF5 and resultant MAGD signaled by hyperstabilized microtubules may be involved in the tumor suppression activity of RASSF1A, a natural microtubule stabilizer and interaction partner with C19ORF5, and the taxoid drug family.
Figures



Similar articles
-
Putative tumor suppressor RASSF1 interactive protein and cell death inducer C19ORF5 is a DNA binding protein.Biochem Biophys Res Commun. 2005 Jul 8;332(3):670-6. doi: 10.1016/j.bbrc.2005.05.006. Biochem Biophys Res Commun. 2005. PMID: 15907802 Free PMC article.
-
Specificity of the methylation-suppressed A isoform of candidate tumor suppressor RASSF1 for microtubule hyperstabilization is determined by cell death inducer C19ORF5.Cancer Res. 2005 Mar 1;65(5):1830-8. doi: 10.1158/0008-5472.CAN-04-3896. Cancer Res. 2005. PMID: 15753381
-
RASSF1A interacts with microtubule-associated proteins and modulates microtubule dynamics.Cancer Res. 2004 Jun 15;64(12):4112-6. doi: 10.1158/0008-5472.CAN-04-0267. Cancer Res. 2004. PMID: 15205320
-
Dual function microtubule- and mitochondria-associated proteins mediate mitotic cell death.Cell Oncol. 2009;31(5):393-405. doi: 10.3233/CLO-2009-0484. Cell Oncol. 2009. PMID: 19759419 Free PMC article.
-
Microtubules, mitochondria, and molecular chaperones: a new hypothesis for in vivo assembly of microtubules.Biochem Cell Biol. 1990 Dec;68(12):1352-63. doi: 10.1139/o90-198. Biochem Cell Biol. 1990. PMID: 1982213 Review.
Cited by
-
The Role and Function of Ras-association domain family in Cancer: A Review.Genes Dis. 2019 Jul 27;6(4):378-384. doi: 10.1016/j.gendis.2019.07.008. eCollection 2019 Dec. Genes Dis. 2019. PMID: 31832517 Free PMC article. Review.
-
Microtubule stability, Golgi organization, and transport flux require dystonin-a2-MAP1B interaction.J Cell Biol. 2012 Mar 19;196(6):727-42. doi: 10.1083/jcb.201107096. Epub 2012 Mar 12. J Cell Biol. 2012. PMID: 22412020 Free PMC article.
-
Robust autophagy/mitophagy persists during mitosis.Cell Cycle. 2009 May 15;8(10):1616-20. doi: 10.4161/cc.8.10.8577. Epub 2009 May 27. Cell Cycle. 2009. PMID: 19411827 Free PMC article.
-
Role of Microtubule-Associated Protein 1b in Urothelial Carcinoma: Overexpression Predicts Poor Prognosis.Cancers (Basel). 2020 Mar 9;12(3):630. doi: 10.3390/cancers12030630. Cancers (Basel). 2020. PMID: 32182788 Free PMC article.
-
Mimitin - a novel cytokine-regulated mitochondrial protein.BMC Cell Biol. 2009 Mar 31;10:23. doi: 10.1186/1471-2121-10-23. BMC Cell Biol. 2009. PMID: 19331698 Free PMC article.
References
-
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9. - PubMed
-
- Duesberg P, Rasnick D. Aneuploidy, the somatic mutation that makes cancer a species of its own. Cell Motil Cytoskeleton. 2000;47:81–107. - PubMed
-
- Jallepalli PV, Lengauer C. Chromosome segregation and cancer: cutting through the mystery. Nat Rev Cancer. 2001;1:109–17. - PubMed
-
- Schneider BL, Kulesz-Martin M. Destructive cycles: the role of genomic instability and adaptation in carcino-genesis. Carcinogenesis. 2004;25:2033–44. - PubMed
-
- Sorger PK, Dobles M, Tournebize R, Hyman AA. Coupling cell division and cell death to microtubule dynamics. Curr Opin Cell Biol. 1997;9:807–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

