Biological and biochemical functions of RNA in the tetrahymena telomerase holoenzyme
- PMID: 15899850
- PMCID: PMC1140614
- DOI: 10.1128/MCB.25.11.4442-4454.2005
Biological and biochemical functions of RNA in the tetrahymena telomerase holoenzyme
Abstract
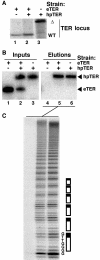
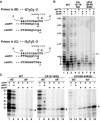
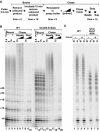
Telomerase extends chromosome ends by the synthesis of tandem simple-sequence repeats. Studies of minimal recombinant telomerase ribonucleoprotein (RNP) reconstituted in vitro have revealed sequences within the telomerase RNA subunit (TER) that are required to establish its internal template and other unique features of enzyme activity. Here we test the significance of these motifs following TER assembly into telomerase holoenzyme in vivo. We established a method for stable expression of epitope-tagged TER and TER variants in place of wild-type Tetrahymena TER. We found that sequence substitutions in nontemplate regions of TER altered telomere length maintenance in vivo, with an increase or decrease in the set point for telomere length homeostasis. We also characterized the in vitro activity of the telomerase holoenzymes reconstituted with TER variants, following RNA-based RNP affinity purification from cell extracts. We found that nontemplate sequence substitutions imposed specific defects in the fidelity and processivity of template use. These findings demonstrate nontemplate functions of TER that are critical for the telomerase holoenzyme catalytic cycle and for proper telomere length maintenance in vivo.
Figures






Similar articles
-
Ciliate telomerase RNA loop IV nucleotides promote hierarchical RNP assembly and holoenzyme stability.RNA. 2010 Mar;16(3):563-71. doi: 10.1261/rna.1936410. Epub 2010 Jan 27. RNA. 2010. PMID: 20106956 Free PMC article.
-
The architecture of Tetrahymena telomerase holoenzyme.Nature. 2013 Apr 11;496(7444):187-92. doi: 10.1038/nature12062. Epub 2013 Apr 3. Nature. 2013. PMID: 23552895 Free PMC article.
-
Shared Subunits of Tetrahymena Telomerase Holoenzyme and Replication Protein A Have Different Functions in Different Cellular Complexes.J Biol Chem. 2017 Jan 6;292(1):217-228. doi: 10.1074/jbc.M116.763664. Epub 2016 Nov 28. J Biol Chem. 2017. PMID: 27895115 Free PMC article.
-
xRRM: a new class of RRM found in the telomerase La family protein p65.RNA Biol. 2013 Mar;10(3):353-9. doi: 10.4161/rna.23608. Epub 2013 Jan 17. RNA Biol. 2013. PMID: 23328630 Free PMC article. Review.
-
Structure and function of telomerase RNA.Curr Opin Struct Biol. 2006 Jun;16(3):307-18. doi: 10.1016/j.sbi.2006.05.005. Epub 2006 May 18. Curr Opin Struct Biol. 2006. PMID: 16713250 Review.
Cited by
-
Disease-associated human telomerase RNA variants show loss of function for telomere synthesis without dominant-negative interference.Mol Cell Biol. 2008 Oct;28(20):6510-20. doi: 10.1128/MCB.00777-08. Epub 2008 Aug 18. Mol Cell Biol. 2008. PMID: 18710936 Free PMC article.
-
Ciliate telomerase RNA loop IV nucleotides promote hierarchical RNP assembly and holoenzyme stability.RNA. 2010 Mar;16(3):563-71. doi: 10.1261/rna.1936410. Epub 2010 Jan 27. RNA. 2010. PMID: 20106956 Free PMC article.
-
Structural basis of template-boundary definition in Tetrahymena telomerase.Nat Struct Mol Biol. 2015 Nov;22(11):883-8. doi: 10.1038/nsmb.3101. Epub 2015 Oct 5. Nat Struct Mol Biol. 2015. PMID: 26436828 Free PMC article.
-
A motif in the vertebrate telomerase N-terminal linker of TERT contributes to RNA binding and telomerase activity and processivity.Structure. 2013 Oct 8;21(10):1870-8. doi: 10.1016/j.str.2013.08.013. Epub 2013 Sep 19. Structure. 2013. PMID: 24055314 Free PMC article.
-
High-resolution physical and functional mapping of the template adjacent DNA binding site in catalytically active telomerase.Proc Natl Acad Sci U S A. 2007 May 22;104(21):8791-6. doi: 10.1073/pnas.0703157104. Epub 2007 May 9. Proc Natl Acad Sci U S A. 2007. PMID: 17494734 Free PMC article.
References
-
- Autexier, C., and C. W. Greider. 1995. Boundary elements of the Tetrahymena telomerase RNA template and alignment domains. Genes Dev. 9:2227-2239. - PubMed
-
- Blackburn, E. H. 2000. The end of the (DNA) line. Nat. Struct. Biol. 7:847-850. - PubMed
-
- Bryan, T. M., K. J. Goodrich, and T. R. Cech. 2000. A mutant of Tetrahymena telomerase reverse transcriptase with increased processivity. J. Biol. Chem. 275:24199-24207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
