Mice deficient in oocyte-specific oligoadenylate synthetase-like protein OAS1D display reduced fertility
- PMID: 15899864
- PMCID: PMC1140637
- DOI: 10.1128/MCB.25.11.4615-4624.2005
Mice deficient in oocyte-specific oligoadenylate synthetase-like protein OAS1D display reduced fertility
Abstract
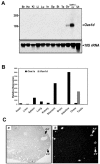
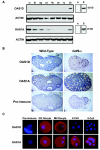
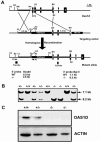
The double-stranded RNA (dsRNA)-induced interferon response is a defense mechanism against viral infection. Upon interferon activation by dsRNA, 2',5'-oligoadenylate synthetase 1 (OAS1A) is induced; it binds dsRNA and converts ATP into 2',5'-linked oligomers of adenosine (called 2-5A), which activate RNase L that in turn degrades viral and cellular RNAs. In a screen to identify oocyte-specific genes, we identified a novel murine cDNA encoding an ovary-specific 2',5'-oligoadenylate synthetase-like protein, OAS1D, which displays 59% identity with OAS1A. OAS1D is predominantly cytoplasmic and is exclusively expressed in growing oocytes and early embryos. Like OAS1A, OAS1D binds the dsRNA mimetic poly(I-C), but unlike OAS1A, it lacks 2'-5' adenosine linking activity. OAS1D interacts with OAS1A and inhibits the enzymatic activity of OAS1A. Mutant mice lacking OAS1D (Oas1d(-/-)) display reduced fertility due to defects in ovarian follicle development, decreased efficiency of ovulation, and eggs that are fertilized arrest at the one-cell stage. These effects are exacerbated after activation of the interferon/OAS1A/RNase L pathway by poly(I-C). We propose that OAS1D suppresses the interferon/OAS/RNase L-mediated cellular destruction by interacting with OAS1A during oogenesis and early embryonic development.
Figures



Similar articles
-
RNase L Antiviral Activity Is Not a Critical Component of the Oas1b-Mediated Flavivirus Resistance Phenotype.J Virol. 2019 Oct 29;93(22):e00946-19. doi: 10.1128/JVI.00946-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462564 Free PMC article.
-
Activation of RNase L by Murine Coronavirus in Myeloid Cells Is Dependent on Basal Oas Gene Expression and Independent of Virus-Induced Interferon.J Virol. 2016 Jan 6;90(6):3160-72. doi: 10.1128/JVI.03036-15. J Virol. 2016. PMID: 26739051 Free PMC article.
-
The role of mouse 2',5'-oligoadenylate synthetase 1 paralogs.Infect Genet Evol. 2016 Nov;45:393-401. doi: 10.1016/j.meegid.2016.09.018. Epub 2016 Sep 21. Infect Genet Evol. 2016. PMID: 27663720
-
The oligoadenylate synthetase family: an ancient protein family with multiple antiviral activities.J Interferon Cytokine Res. 2011 Jan;31(1):41-7. doi: 10.1089/jir.2010.0107. Epub 2010 Dec 12. J Interferon Cytokine Res. 2011. PMID: 21142819 Review.
-
The human 2',5'-oligoadenylate synthetase family: interferon-induced proteins with unique enzymatic properties.J Interferon Cytokine Res. 1999 Apr;19(4):295-308. doi: 10.1089/107999099313992. J Interferon Cytokine Res. 1999. PMID: 10334380 Review.
Cited by
-
Gene expression during the oocyte-to-embryo transition in mammals.Mol Reprod Dev. 2009 Sep;76(9):805-18. doi: 10.1002/mrd.21038. Mol Reprod Dev. 2009. PMID: 19363788 Free PMC article. Review.
-
The power of mouse genetics to study spermatogenesis.J Androl. 2010 Jan-Feb;31(1):34-44. doi: 10.2164/jandrol.109.008227. Epub 2009 Oct 29. J Androl. 2010. PMID: 19875488 Free PMC article. Review.
-
Identification of potential biomarkers in donor cows for in vitro embryo production by granulosa cell transcriptomics.PLoS One. 2017 Apr 12;12(4):e0175464. doi: 10.1371/journal.pone.0175464. eCollection 2017. PLoS One. 2017. PMID: 28403200 Free PMC article.
-
The Flvr-encoded murine oligoadenylate synthetase 1b (Oas1b) suppresses 2-5A synthesis in intact cells.Virology. 2011 Jan 20;409(2):262-70. doi: 10.1016/j.virol.2010.10.016. Epub 2010 Nov 5. Virology. 2011. PMID: 21056894 Free PMC article.
-
Cloning and expression profiling of small RNAs expressed in the mouse ovary.RNA. 2007 Dec;13(12):2366-80. doi: 10.1261/rna.754207. Epub 2007 Oct 19. RNA. 2007. PMID: 17951331 Free PMC article.
References
-
- Burke, D. C., C. F. Graham, and J. M. Lehman. 1978. Appearance of interferon inducibility and sensitivity during differentiation of murine teratocarcinoma cells in vitro. Cell 13:243-248. - PubMed
-
- Burns, K. H., M. M. Viveiros, Y. Ren, P. Wang, F. J. DeMayo, D. E. Frail, J. J. Eppig, and M. M. Matzuk. 2003. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science 300:633-636. - PubMed
-
- Carpten, J., N. Nupponen, S. Isaacs, R. Sood, C. Robbins, J. Xu, M. Faruque, T. Moses, C. Ewing, E. Gillanders, P. Hu, P. Bujnovszky, I. Makalowska, A. Baffoe-Bonnie, D. Faith, J. Smith, D. Stephan, K. Wiley, M. Brownstein, D. Gildea, B. Kelly, R. Jenkins, G. Hostetter, M. Matikainen, J. Schleutker, K. Klinger, T. Connors, Y. Xiang, Z. Wang, A. De Marzo, N. Papadopoulos, O. P. Kallioniemi, R. Burk, D. Meyers, H. Gronberg, P. Meltzer, R. Silverman, J. Bailey-Wilson, P. Walsh, W. Isaacs, and J. Trent. 2002. Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nat. Genet. 30:181-184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
