Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue
- PMID: 15899981
- PMCID: PMC1129148
- DOI: 10.1073/pnas.0502761102
Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue
Abstract
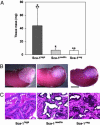
We previously showed that prostatic stem cells are concentrated in the proximal regions of prostatic ducts. We now report that these stem cells can be purified from isolated proximal duct regions by virtue of their high expression of the cell surface protein stem cell antigen 1 (Sca-1). In an in vivo prostate reconstitution assay, the purified Sca-1-expressing cell population isolated from the proximal region of ducts was more effective in generating prostatic tissue than a comparable population of Sca-1-depleted cells (203.0 +/- 83.1 mg vs. 11.9 +/- 9.2 mg) or a population of Sca-1-expressing cells isolated from the remaining regions of ducts (transit-amplifying cells) (31.9 +/- 24.1 mg). Almost all of the proliferative capacity of the proximal duct Sca-1-expressing cell population resides within the fraction of cells that express high levels of Sca-1 (top one-third), with the proximal region of prostatic ducts containing 7.2-fold more Sca-1(high) cells than the remaining regions. More than 60% of the high-expressing cells coexpress alpha6 integrin and the anti-apoptotic factor Bcl-2, markers that are also characteristic of stem cells of other origins. Further stratification of the phenotype of the stem cells may enable the development of rational therapies for treating prostate cancer and benign prostatic hyperplasia.
Figures



References
-
- Al-Hajj, M. & Clarke, M. F. (2004) Oncogene 23, 7274-7282. - PubMed
-
- Lapidot, T., Sirard, C., Vormoor, J., Murdoch, B., Hoang, T., Caceres-Cortes, J., Minden, M., Paterson, B., Caligiuri, M. A. & Dick, J. E. (1994) Nature 367, 645-648. - PubMed
-
- Pardal, R., Clarke, M. F. & Morrison, S. J. (2003) Nat. Rev. Cancer 3, 895-902. - PubMed
-
- Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. (2001) Nature 414, 105-111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

