An overview of menopausal oestrogen-progestin hormone therapy and breast cancer risk
- PMID: 15900297
- PMCID: PMC2361783
- DOI: 10.1038/sj.bjc.6602617
An overview of menopausal oestrogen-progestin hormone therapy and breast cancer risk
Abstract
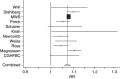
Results from the Women's Health Initiative (WHI) trial support findings from observational studies that oestrogen-progestin therapy (EPT) use is associated with an increase in breast cancer risk. We conducted a meta-analysis using EPT-specific results from the Collaborative Group on Hormonal Factors in Breast Cancer (CGHFBC) pooled analysis and studies published since that report to obtain an overview of EPT use and breast cancer risk. We also assessed risk by histologic subtype of breast cancer, by schedule of the progestin component of EPT, and by recency of use. We estimate that overall, EPT results in a 7.6% increase in breast cancer risk per year of use. The risk was statistically significantly lower in US studies than in European studies - 5.2 vs 7.9%. There was a significantly higher risk for continuous-combined than for sequential EPT use in Scandinavian studies where much higher total doses of progestin were used in continuous-combined than in sequential EPT. We observed no overall difference in risk for lobular vs ductal carcinoma but did observe a slightly higher risk for current vs past EPT use.
Figures
References
-
- Back DJ, Bates M, Breckenridge AM, Hall JM, MacIver M, Orme ML, Park BK, Rowe PH (1981) The pharmacokinetics of levonorgestrel and ethynylestradiol in women – studies with Ovran and Ovranette. Contraception 23: 229–239 - PubMed
-
- Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101 - PubMed
-
- Chen CL, Weiss NS, Newcomb P, Barlow W, White E (2002) Hormone replacement therapy in relation to breast cancer. JAMA 287: 734–741 - PubMed
-
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A (2003) Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA 289: 3243–3253 - PubMed
-
- Collaborative Group on Hormonal Factors in Breast Cancer (1997) Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52 705 women with breast cancer and 108 411 women without breast cancer. Lancet 350: 1047–1059 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous