Intravital observation of Plasmodium berghei sporozoite infection of the liver
- PMID: 15901208
- PMCID: PMC1135295
- DOI: 10.1371/journal.pbio.0030192
Intravital observation of Plasmodium berghei sporozoite infection of the liver
Abstract
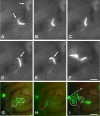
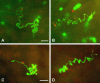
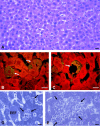
Plasmodium sporozoite invasion of liver cells has been an extremely elusive event to study. In the prevailing model, sporozoites enter the liver by passing through Kupffer cells, but this model was based solely on incidental observations in fixed specimens and on biochemical and physiological data. To obtain direct information on the dynamics of sporozoite infection of the liver, we infected live mice with red or green fluorescent Plasmodium berghei sporozoites and monitored their behavior using intravital microscopy. Digital recordings show that sporozoites entering a liver lobule abruptly adhere to the sinusoidal cell layer, suggesting a high-affinity interaction. They glide along the sinusoid, with or against the bloodstream, to a Kupffer cell, and, by slowly pushing through a constriction, traverse across the space of Disse. Once inside the liver parenchyma, sporozoites move rapidly for many minutes, traversing several hepatocytes, until ultimately settling within a final one. Migration damage to hepatocytes was confirmed in liver sections, revealing clusters of necrotic hepatocytes adjacent to structurally intact, sporozoite-infected hepatocytes, and by elevated serum alanine aminotransferase activity. In summary, malaria sporozoites bind tightly to the sinusoidal cell layer, cross Kupffer cells, and leave behind a trail of dead hepatocytes when migrating to their final destination in the liver.
Figures








References
-
- Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilization of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–996. - PubMed
-
- Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, et al. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992;70:1021–1033. - PubMed
-
- Ying P, Shakibaei M, Patankar MS, Clavijo P, Beavis RC, et al. The malaria circumsporozoite protein: Interaction of the conserved regions I and II-plus with heparin-like oligosaccharides in heparan sulfate. Exp Parasitol. 1997;85:168–182. - PubMed
-
- Pradel G, Garapaty S, Frevert U. Proteoglycans mediate malaria sporozoite targeting to the liver. Mol Microbiol. 2002;45:637–651. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

