The signal peptide of the rat corticotropin-releasing factor receptor 1 promotes receptor expression but is not essential for establishing a functional receptor
- PMID: 15901239
- PMCID: PMC1198925
- DOI: 10.1042/BJ20050113
The signal peptide of the rat corticotropin-releasing factor receptor 1 promotes receptor expression but is not essential for establishing a functional receptor
Abstract
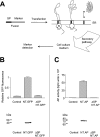
Approximately 5-10% of the GPCRs (G-protein-coupled receptors) contain N-terminal signal peptides that are cleaved off during receptor insertion into the ER (endoplasmic reticulum) membrane by the signal peptidases of the ER. The reason as to why only a subset of GPCRs requires these additional signal peptides is not known. We have recently shown that the signal peptide of the human ET(B)-R (endothelin B receptor) does not influence receptor expression but is necessary for the translocation of the receptor's N-tail across the ER membrane and thus for the establishment of a functional receptor [Köchl, Alken, Rutz, Krause, Oksche, Rosenthal and Schülein (2002) J. Biol. Chem. 277, 16131-16138]. In the present study, we show that the signal peptide of the rat CRF-R1 (corticotropin-releasing factor receptor 1) has a different function: a mutant of the CRF-R1 lacking the signal peptide was functional and displayed wild-type properties with respect to ligand binding and activation of adenylate cyclase. However, immunoblot analysis and confocal laser scanning microscopy revealed that the mutant receptor was expressed at 10-fold lower levels than the wild-type receptor. Northern-blot and in vitro transcription translation analyses precluded the possibility that the reduced receptor expression is due to decreased transcription or translation levels. Thus the signal peptide of the CRF-R1 promotes an early step of receptor biogenesis, such as targeting of the nascent chain to the ER membrane and/or the gating of the protein-conducting translocon of the ER membrane.
Figures






References
-
- Wallin E., von Heijne G. Properties of N-terminal tails in G protein-coupled receptors: a statistical study. Protein Eng. 1995;8:693–698. - PubMed
-
- Köchl R., Alken M., Rutz C., Krause G., Oksche A., Rosenthal W., Schülein R. The signal peptide of the G protein-coupled human endothelin B receptor is necessary for translocation of the N terminal tail across the endoplasmic reticulum membrane. J. Biol. Chem. 2002;277:16131–16138. - PubMed
-
- Rapoport T. A., Jungnickel B., Kutay U. Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu. Rev. Biochem. 1996;65:271–303. - PubMed
-
- Hegde R. S., Lingappa V. R. Regulation of protein biogenesis at the endoplasmic reticulum membrane. Trends Cell Biol. 1999;9:132–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

