Demonstration of a glycoprotein derived from the Ceacam10 gene in mouse seminal vesicle secretions
- PMID: 15901639
- PMCID: PMC7109865
- DOI: 10.1095/biolreprod.105.039651
Demonstration of a glycoprotein derived from the Ceacam10 gene in mouse seminal vesicle secretions
Abstract
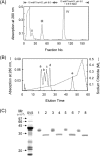
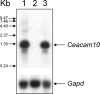
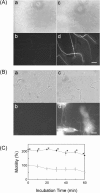
CEACAM10 was purified from mouse seminal vesicle secretions by a series of purification steps that included ion exchange chromatography on a DEAE-Sephacel column and ion exchange high-performance liquid chromatography on a sulfopropyl column. It was shown to be a 36-kDa glycoprotein with an N-linked carbohydrate moiety. The circular dichromoism spectrum of CEACAM10 in 50 mM phosphate buffer at pH 7.4 appeared as one negative band arising from the beta form at 217 nm. CEACAM10 was expressed predominantly in seminal vesicles of adult mice. Both CEACAM10 and its mRNA were demonstrated on the luminal epithelium of the mucosal folds in the seminal vesicle. The amount of Ceacam10 mRNA in the seminal vesicle was correlated with the stage of animal maturation. Castration of adult mice resulted in cessation of Ceacam10 expression, while treatment of castrated mice with testosterone propionate in corn oil restored Ceacam10 expression in the seminal vesicle. During the entire course of pregnancy, Ceacam10 might be silent in the embryo. A cytochemical study illustrated the presence of the CEACAM10 binding region on the entire surface of mouse sperm. CEACAM10-sperm binding greatly enhanced sperm motility in vitro.
Figures




Similar articles
-
Developmental profile of a caltrin-like protease inhibitor, P12, in mouse seminal vesicle and characterization of its binding sites on sperm surface.Biol Reprod. 1998 Dec;59(6):1498-505. doi: 10.1095/biolreprod59.6.1498. Biol Reprod. 1998. PMID: 9828198
-
SSLP-1, a secreted Ly-6 protein purified from mouse seminal vesicle fluid.Reproduction. 2006 Sep;132(3):493-500. doi: 10.1530/rep.1.01183. Reproduction. 2006. PMID: 16940290
-
SPINKL, a Kazal-type serine protease inhibitor-like protein purified from mouse seminal vesicle fluid, is able to inhibit sperm capacitation.Reproduction. 2008 Nov;136(5):559-71. doi: 10.1530/REP-07-0375. Epub 2008 Aug 20. Reproduction. 2008. PMID: 18715980
-
cis-elements required for expression of human protein C inhibitor gene in HepG2 cells and its androgen-dependent expression in rat reproductive organs.Semin Thromb Hemost. 2000;26(1):75-83. doi: 10.1055/s-2000-9807. Semin Thromb Hemost. 2000. PMID: 10805286 Review.
-
Epididymal Binder of SPerm genes and proteins: what do we know a decade later?Andrology. 2015 Sep;3(5):817-24. doi: 10.1111/andr.12089. Epub 2015 Jul 31. Andrology. 2015. PMID: 26236016 Review.
Cited by
-
Secretory mouse quiescin sulfhydryl oxidase 1 aggregates defected human and mouse spermatozoa in vitro and in vivo.iScience. 2021 Sep 25;24(10):103167. doi: 10.1016/j.isci.2021.103167. eCollection 2021 Oct 22. iScience. 2021. PMID: 34667943 Free PMC article.
-
Sperm competition risk drives plasticity in seminal fluid composition.BMC Biol. 2015 Oct 27;13:87. doi: 10.1186/s12915-015-0197-2. BMC Biol. 2015. PMID: 26507392 Free PMC article.
-
Polarized epithelium-sperm co-culture system reveals stimulatory factors for the secretion of mouse epididymal quiescin sulfhydryl oxidase 1.J Reprod Dev. 2022 Jun 1;68(3):198-208. doi: 10.1262/jrd.2021-128. Epub 2022 Feb 26. J Reprod Dev. 2022. PMID: 35228412 Free PMC article.
-
Identification, characterization and purification of porcine Quiescin Q6-Sulfydryl Oxidase 2 protein.BMC Vet Res. 2017 Jun 29;13(1):205. doi: 10.1186/s12917-017-1125-1. BMC Vet Res. 2017. PMID: 28662655 Free PMC article.
-
Heterogenous turnover of sperm and seminal vesicle proteins in the mouse revealed by dynamic metabolic labeling.Mol Cell Proteomics. 2012 Jun;11(6):M111.014993. doi: 10.1074/mcp.M111.014993. Epub 2012 Feb 13. Mol Cell Proteomics. 2012. PMID: 22331477 Free PMC article.
References
-
- Nichol R, Hunter RH, de Lamirande E, Gagnon C, Cooke GM. Motility of spermatozoa in hydrosalpingeal and follicular fluid of pigs. J Reprod Fertil 199710:79–86 - PubMed
-
- Iwamoto T, Tsang A, Luterman M, Dickson J, de Lamirande E, Okuno M, Mohri H, Gagnon C. Purification and characterization of a sperm motility-dynein ATPase inhibitor from boar seminal plasma. Mol Reprod Dev 199231:55–62 - PubMed
-
- Jeng H, Liu KM, Chang WC. Purification and characterization of reversible sperm motility inhibitors from porcine seminal plasma. Biochem Biophys Res Commun 1993191:435–440 - PubMed
-
- Al-Somai N, Vishwanath R, Shannon P, Molan PC. Low molecular weight components in bovine semen diffusate and their effects on motility of bull sperm. Reprod Fertil Dev 19946:165–171 - PubMed
-
- Peitz B. Effects of seminal vesicle fluid components on sperm motility in the house mouse. J Reprod Fertil 198883:169–176 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

