Xema, a foxi-class gene expressed in the gastrula stage Xenopus ectoderm, is required for the suppression of mesendoderm
- PMID: 15901660
- PMCID: PMC3525708
- DOI: 10.1242/dev.01865
Xema, a foxi-class gene expressed in the gastrula stage Xenopus ectoderm, is required for the suppression of mesendoderm
Abstract
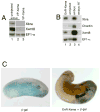
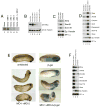
The molecular basis of vertebrate germ layer formation has been the focus of intense scrutiny for decades, and the inductive interactions underlying this process are well defined. Only recently, however, have studies demonstrated that the regulated inhibition of ectopic germ layer formation is also crucial for patterning the early vertebrate embryo. We report here the characterization of Xema (Xenopus Ectodermally-expressed Mesendoderm Antagonist), a novel member of the Foxi-subclass of winged-helix transcription factors that is involved in the suppression of ectopic germ layer formation in the frog, Xenopus laevis. Xema transcripts are restricted to the animal pole ectoderm during early Xenopus development. Ectopic expression of Xema RNA inhibits mesoderm induction, both by growth factors and in the marginal zone, in vivo. Conversely, introduction of antisense morpholino oligonucleotides directed against the Xema transcript stimulates the expression of a broad range of mesodermal and endodermal marker genes in the animal pole. Our studies demonstrate that Xema is both necessary and sufficient for the inhibition of ectopic mesendoderm in the cells of the presumptive ectoderm, and support a model in which Fox proteins function in part to restrict inappropriate germ layer development throughout the vertebrate embryo.
Figures



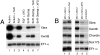
Similar articles
-
Expression cloning of Xenopus Os4, an evolutionarily conserved gene, which induces mesoderm and dorsal axis.Dev Biol. 2001 Nov 1;239(1):118-31. doi: 10.1006/dbio.2001.0420. Dev Biol. 2001. PMID: 11784023
-
Cell migration in the Xenopus gastrula.Wiley Interdiscip Rev Dev Biol. 2018 Nov;7(6):e325. doi: 10.1002/wdev.325. Epub 2018 Jun 26. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 29944210 Review.
-
A novel role for Ascl1 in the regulation of mesendoderm formation via HDAC-dependent antagonism of VegT.Development. 2016 Feb 1;143(3):492-503. doi: 10.1242/dev.126292. Epub 2015 Dec 23. Development. 2016. PMID: 26700681 Free PMC article.
-
Lens and retina formation require expression of Pitx3 in Xenopus pre-lens ectoderm.Dev Dyn. 2005 Nov;234(3):577-89. doi: 10.1002/dvdy.20540. Dev Dyn. 2005. PMID: 16170783
-
Repression of Inappropriate Gene Expression in the Vertebrate Embryonic Ectoderm.Genes (Basel). 2019 Nov 6;10(11):895. doi: 10.3390/genes10110895. Genes (Basel). 2019. PMID: 31698780 Free PMC article. Review.
Cited by
-
Identification of a novel negative regulator of activin/nodal signaling in mesendodermal formation of Xenopus embryos.J Biol Chem. 2009 Jun 19;284(25):17052-17060. doi: 10.1074/jbc.M109.007443. Epub 2009 Apr 22. J Biol Chem. 2009. PMID: 19389709 Free PMC article.
-
Microarray identification of novel genes downstream of Six1, a critical factor in cranial placode, somite, and kidney development.Dev Dyn. 2015 Feb;244(2):181-210. doi: 10.1002/dvdy.24229. Epub 2014 Dec 16. Dev Dyn. 2015. PMID: 25403746 Free PMC article.
-
Foxi1 regulates multiple steps of mucociliary development and ionocyte specification through transcriptional and epigenetic mechanisms.bioRxiv [Preprint]. 2024 Nov 4:2024.10.27.620464. doi: 10.1101/2024.10.27.620464. bioRxiv. 2024. PMID: 39484493 Free PMC article. Preprint.
-
Regulation of vertebrate embryogenesis by the exon junction complex core component Eif4a3.Dev Dyn. 2010 Jul;239(7):1977-87. doi: 10.1002/dvdy.22330. Dev Dyn. 2010. PMID: 20549732 Free PMC article.
-
Endogenous ROS production in early differentiation state suppresses endoderm differentiation via transient FOXC1 expression.Cell Death Discov. 2022 Apr 1;8(1):150. doi: 10.1038/s41420-022-00961-2. Cell Death Discov. 2022. PMID: 35365611 Free PMC article.
References
-
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–70. - PubMed
-
- Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–74. - PubMed
-
- Bassez T, Paris J, Omilli F, Dorel C, Osborne HB. Post-transcriptional regulation of ornithine decarboxylase in Xenopus laevis oocytes. Development. 1990;110:955–62. - PubMed
-
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. - PubMed
-
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

