PAX6 expression identifies progenitor cells for corneal keratocytes
- PMID: 15901670
- PMCID: PMC2876310
- DOI: 10.1096/fj.04-2770fje
PAX6 expression identifies progenitor cells for corneal keratocytes
Abstract
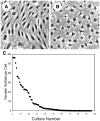
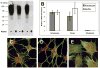
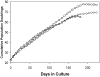
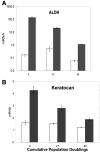
Keratocytes of the corneal stroma produce a transparent extracellular matrix required for vision. During wound-healing and in vitro, keratocytes proliferate, becoming fibroblastic, and lose biosynthesis of unique corneal matrix components. This study sought identification of cells in the corneal stroma capable of assuming a keratocyte phenotype after extensive proliferation. About 3% of freshly isolated bovine stromal cells exhibited clonal growth. In low-mitogen media, selected clonal cultures displayed dendritic morphology and expressed high levels of keratan sulfate, aldehyde dehydrogenase 3A1, and keratocan, molecular markers of keratocyte phenotype. In protein-free media, both primary keratocytes and selected clonal cells aggregated to form attachment-independent spheroids expressing elevated levels of those marker molecules. The selected clonal cells exhibited normal karyotype and underwent replicative senescence after 65-70 population doublings; however, they continued expression of keratocyte phenotypic markers throughout their replicative life span. The progenitor cells expressed elevated mRNA for several genes characteristic of stem cells and also for genes expressed during ocular development PAX6, Six2, and Six3. PAX6 protein was detected in the cultured progenitor cells and a small number of stromal cells in intact tissue but was absent in cultured keratocytes and fibroblasts. Cytometry demonstrated PAX6 protein in 4% of freshly isolated stromal cells. These results demonstrate the presence of a previously unrecognized population of PAX6-positive cells in adult corneal stroma that maintain the potential to assume a keratocyte phenotype even after extensive replication. The presence of such progenitor cells has implications for corneal biology and for cell-based therapies targeting corneal scarring.
Figures


Similar articles
-
Multipotent stem cells in human corneal stroma.Stem Cells. 2005 Oct;23(9):1266-75. doi: 10.1634/stemcells.2004-0256. Epub 2005 Jul 28. Stem Cells. 2005. PMID: 16051989 Free PMC article.
-
Increased apoptosis and abnormal wound-healing responses in the heterozygous Pax6+/- mouse cornea.Invest Ophthalmol Vis Sci. 2006 May;47(5):1911-7. doi: 10.1167/iovs.05-1028. Invest Ophthalmol Vis Sci. 2006. PMID: 16638998
-
Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells.Stem Cells. 2009 Mar;27(3):642-52. doi: 10.1634/stemcells.2008-0721. Stem Cells. 2009. PMID: 19074417 Free PMC article.
-
Corneal epithelial stem cells: deficiency and regulation.Stem Cell Rev. 2008 Sep;4(3):159-68. doi: 10.1007/s12015-008-9029-x. Epub 2008 Jul 12. Stem Cell Rev. 2008. PMID: 18622724 Review.
-
The molecular basis of corneal transparency.Exp Eye Res. 2010 Sep;91(3):326-35. doi: 10.1016/j.exer.2010.06.021. Epub 2010 Jul 3. Exp Eye Res. 2010. PMID: 20599432 Free PMC article. Review.
Cited by
-
Adipose-derived stem cells integrate into trabecular meshwork with glaucoma treatment potential.FASEB J. 2020 May;34(5):7160-7177. doi: 10.1096/fj.201902326R. Epub 2020 Apr 7. FASEB J. 2020. PMID: 32259357 Free PMC article.
-
Lacrimal Gland Repair Using Progenitor Cells.Stem Cells Transl Med. 2017 Jan;6(1):88-98. doi: 10.5966/sctm.2016-0191. Epub 2016 Aug 15. Stem Cells Transl Med. 2017. PMID: 28170196 Free PMC article.
-
Sphere formation from corneal keratocytes and phenotype specific markers.Exp Eye Res. 2011 Dec;93(6):898-905. doi: 10.1016/j.exer.2011.10.004. Epub 2011 Oct 21. Exp Eye Res. 2011. PMID: 22032988 Free PMC article.
-
Differentiation Capacity of Human Mesenchymal Stem Cells into Keratocyte Lineage.Invest Ophthalmol Vis Sci. 2019 Jul 1;60(8):3013-3023. doi: 10.1167/iovs.19-27008. Invest Ophthalmol Vis Sci. 2019. PMID: 31310658 Free PMC article.
-
Easy xeno-free and feeder-free method for isolating and growing limbal stromal and epithelial stem cells of the human cornea.PLoS One. 2017 Nov 17;12(11):e0188398. doi: 10.1371/journal.pone.0188398. eCollection 2017. PLoS One. 2017. PMID: 29149196 Free PMC article.
References
-
- Connon CJ, Siegler V, Meek KM, Hodson SA, Caterson B, Kinoshita S, Quantock AJ. Proteoglycan alterations and collagen reorganisation in the secondary avian cornea during development. Ophthalmic Res. 2003;35:177–184. - PubMed
-
- Liu CY, Birk DE, Hassell JR, Kane B, Kao WW. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003;278:21672–21677. - PubMed
-
- Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36:2557–2567. - PubMed
-
- Moller-Pedersen T, Li HF, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998;39:487–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

