Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish
- PMID: 15901770
- PMCID: PMC6724860
- DOI: 10.1523/JNEUROSCI.0679-05.2005
Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish
Abstract
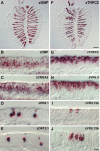
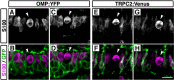
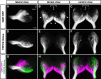
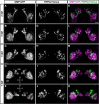
The olfactory epithelium of fish contains two major types of olfactory sensory neurons (OSNs) that are distinct morphologically (ciliated vs microvillous) and possibly functionally. Here, we found that these OSNs express different sets of signal transduction machineries: the ciliated OSNs express OR-type odorant receptors, cyclic nucleotide-gated channel A2 subunit, and olfactory marker protein (OMP), whereas the microvillous OSNs express V2R-type receptors and transient receptor potential channel C2 (TRPC2). To visualize patterns of axonal projection from the two types of OSNs to the olfactory bulb (OB), we generated transgenic zebrafish in which spectrally distinct fluorescent proteins are expressed in the ciliated and microvillous OSNs under the control of OMP and TRPC2 gene promoters, respectively. An observation of whole-mount OB in adult double-transgenic zebrafish revealed that the ciliated OSNs project axons mostly to the dorsal and medial regions of the OB, whereas the microvillous OSNs project axons to the lateral region of the OB. A careful histological examination of OB sections clarified that the axons from the two distinct types of OSNs target different glomeruli in a mutually exclusive manner. This segregation is already established at very early developmental stages in zebrafish embryos. These findings clearly demonstrate the relationships among cell morphology, molecular signatures, and axonal terminations of the two distinct types of OSNs and suggest that the two segregated neural pathways are responsible for coding and processing of different types of odor information in the zebrafish olfactory system.
Figures



References
-
- Axel R (1995) The molecular logic of smell. Sci Am 273: 154-159. - PubMed
-
- Barth AL, Justice NJ, Ngai J (1996) Asynchronous onset of odorant receptor expression in the developing zebrafish olfactory system. Neuron 16: 23-34. - PubMed
-
- Barth AL, Dugas JC, Ngai J (1997) Noncoordinate expression of odorant receptor genes tightly linked in the zebrafish genome. Neuron 19: 359-369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
