Regulation of activin mRNA and Smad2 phosphorylation by antidepressant treatment in the rat brain: effects in behavioral models
- PMID: 15901772
- PMCID: PMC6724846
- DOI: 10.1523/JNEUROSCI.5155-04.2005
Regulation of activin mRNA and Smad2 phosphorylation by antidepressant treatment in the rat brain: effects in behavioral models
Abstract
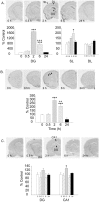
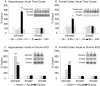
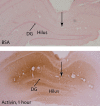
Activin is a member of the transforming growth factor-beta family that is involved in cell differentiation, hormone secretion, and regulation of neuron survival. The cellular responses to activin are mediated by phosphorylation of a downstream target, Smad2. The current study examines the influence of chronic electroconvulsive seizures (ECSs), as well as chemical antidepressants, on the expression of activin betaA and the phosphorylation of Smad2 in the rat hippocampus and frontal cortex. Chronic ECSs (10 d) resulted in a significant increase in activin betaA mRNA expression and Smad2 phosphorylation in both the hippocampus and frontal cortex. Chronic fluoxetine did not influence activin betaA expression, but fluoxetine as well as desipramine did increase Smad2 phosphorylation in the frontal cortex. The functional significance of increased activin was further tested by examining the effects of activin infusions into the hippocampus on a behavioral model of depression, the forced swim test (FST). A single bilateral infusion of activin A or activin B into the dentate gyrus of the hippocampus produced an antidepressant-like effect in the FST that was comparable in magnitude with fluoxetine. In contrast, infusion of the activin antagonist inhibin A did not influence behavior but blocked the effect of activin A. The results suggest that regulation of activin and Smad signaling may contribute to the actions of antidepressant treatment and may represent novel targets for antidepressant drug development.
Figures





Similar articles
-
The delta-opioid receptor agonist (+)BW373U86 regulates BDNF mRNA expression in rats.Neuropsychopharmacology. 2004 Apr;29(4):649-59. doi: 10.1038/sj.npp.1300345. Neuropsychopharmacology. 2004. PMID: 14647482
-
Effects of activin A and its downstream ERK1/2 in oxygen and glucose deprivation after isoflurane-induced postconditioning.Biomed Pharmacother. 2016 Dec;84:535-543. doi: 10.1016/j.biopha.2016.09.075. Epub 2016 Sep 28. Biomed Pharmacother. 2016. PMID: 27693962
-
Activin A, B, and AB increase human trophoblast cell invasion by up-regulating N-cadherin.J Clin Endocrinol Metab. 2014 Nov;99(11):E2216-25. doi: 10.1210/jc.2014-2118. Epub 2014 Aug 8. J Clin Endocrinol Metab. 2014. PMID: 25105734
-
Beta A versus beta B: is it merely a matter of expression?Mol Cell Endocrinol. 2004 Oct 15;225(1-2):9-17. doi: 10.1016/j.mce.2004.02.007. Mol Cell Endocrinol. 2004. PMID: 15451562 Review.
-
Activin Signaling in the Pathogenesis and Therapy of Neuropsychiatric Diseases.Front Mol Neurosci. 2016 May 10;9:32. doi: 10.3389/fnmol.2016.00032. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27242425 Free PMC article. Review.
Cited by
-
Dorsal-Ventral Gradient of Activin Regulates Strength of GABAergic Inhibition along Longitudinal Axis of Mouse Hippocampus in an Activity-Dependent Fashion.Int J Mol Sci. 2023 Aug 24;24(17):13145. doi: 10.3390/ijms241713145. Int J Mol Sci. 2023. PMID: 37685952 Free PMC article.
-
The role of 5-HT receptors in depression.Mol Brain. 2017 Jun 24;10(1):28. doi: 10.1186/s13041-017-0306-y. Mol Brain. 2017. PMID: 28646910 Free PMC article. Review.
-
Association Between FoxO1, A2M, and TGF-β1, Environmental Factors, and Major Depressive Disorder.Front Psychiatry. 2020 Jul 10;11:675. doi: 10.3389/fpsyt.2020.00675. eCollection 2020. Front Psychiatry. 2020. PMID: 32792993 Free PMC article.
-
Research progress on the correlation between transforming growth factor-β level and symptoms of depression.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023 Sep 25;52(5):646-652. doi: 10.3724/zdxbyxb-2023-0269. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37916311 Free PMC article. Review. Chinese, English.
-
Altered response to antidepressant treatment in FoxG1 heterozygous knockout mice.Synapse. 2010 Feb;64(2):169-71. doi: 10.1002/syn.20737. Synapse. 2010. PMID: 19852072 Free PMC article.
References
-
- Andreasson K, Worley PF (1995) Induction of β-A activin expression by synaptic activity and during neocortical development. Neuroscience 69: 781-796. - PubMed
-
- Bengtsson H, Soderstrom S, Ebendal T (1995) Expression of activin receptors type I and II only partially overlaps in the nervous system. NeuroReport 7: 113-116. - PubMed
-
- Bottner M, Krieglstein K, Unsicker K (2000) The transforming growth factor-βs: structure, signaling, and roles in nervous system development and functions. J Neurochem 75: 2227-2240. - PubMed
-
- Cameron VA, Nishimura E, Mathews LS, Lewis KA, Sawchenko PE, Vale WW (1994) Hybridization histochemical localization of activin receptor subtypes in rat brain, pituitary, ovary, and testis. Endocrinology 134: 799-808. - PubMed
-
- Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121: 66-72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
