Adaptation of sensory neurons to hyalectin and decorin proteoglycans
- PMID: 15901777
- PMCID: PMC6724852
- DOI: 10.1523/JNEUROSCI.0773-05.2005
Adaptation of sensory neurons to hyalectin and decorin proteoglycans
Abstract
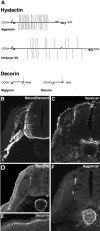
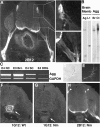
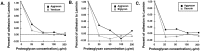
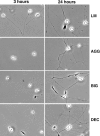
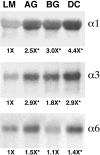
Proteoglycans are abundantly expressed in the pathways of developing and regenerating neurons, yet the responses of neurons to specific proteoglycans are not well characterized. We have shown previously that one chondroitin sulfate proteoglycan (CSPG), aggrecan, is potently inhibitory to sensory axon extension in short-term assays and that over time, embryonic neurons adapt to aggrecan-mediated inhibition through the transcriptional upregulation of integrin expression (Condic et al., 1999). Here, we have compared the response of embryonic sensory neurons to structurally distinct CSPGs that belong to either the hyalectin (or lectican) family of large, aggregating proteoglycans or the decorin (or small leucine-rich proteoglycan) family of smaller proteoglycans. Both of these structurally diverse proteoglycan families are expressed in developing embryos and inhibit outgrowth of embryonic sensory neurons in short-term cultures. These results document a previously uncharacterized inhibitory function for the decorin-family proteoglycan biglycan. Interestingly, embryonic neurons adapt to these diverse proteoglycans over time. Adaptation is associated with upregulation of select integrin alpha subunits in a proteoglycan-specific manner. Overexpression of specific integrin alpha subunits improves neuronal regeneration on some but not all decorin-family CSPGs, suggesting that neurons adapt to inhibition mediated by closely related proteoglycans using distinct mechanisms. Our findings indicate that CSPGs with diverse core proteins and distinct numbers of chondroitin sulfate substitution sites mediate a similar response in sensory neurons, suggesting that increased integrin expression may be an effective means of promoting axonal regeneration in the presence of diverse inhibitory proteoglycan species in vivo.
Figures
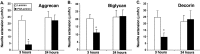

References
-
- Bandtlow CE, Zimmermann DR (2000) Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev 80: 1267-1290. - PubMed
-
- Barberis D, Artigiani S, Casazza A, Corso S, Giordano S, Love CA, Jones EY, Comoglio PM, Tamagnone L (2004) Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J 18: 592-594. - PubMed
-
- Barres BA, Silverstein BE, Corey DP, Chun LL (1988) Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron 1: 791-803. - PubMed
-
- Blaschke UK, Hedbom E, Bruckner P (1996) Distinct isoforms of chicken decorin contain either one or two dermatan sulfate chains. J Biol Chem 271: 30347-30353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
