Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse
- PMID: 15901791
- PMCID: PMC6724856
- DOI: 10.1523/JNEUROSCI.0093-05.2005
Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse
Abstract
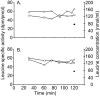
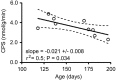
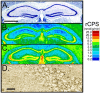
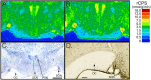
Methylation-induced transcriptional silencing of the fragile X mental retardation-1 (Fmr1) gene leads to absence of the gene product, fragile X mental retardation protein (FMRP), and consequently fragile X syndrome (FrX), an X-linked inherited form of mental retardation. Absence of FMRP in Fmr1 null mice imparts some characteristics of the FrX phenotype, but the precise role of FMRP in neuronal function remains unknown. FMRP is an RNA-binding protein that has been shown to suppress translation of certain mRNAs in vitro. We applied the quantitative autoradiographic L-[1-14C]leucine method to the in vivo determination of regional rates of cerebral protein synthesis (rCPS) in adult wild-type (WT) and Fmr1 null mice at 4 and 6 months of age. Our results show a substantial decrease in rCPS in all brain regions examined between the ages of 4 and 6 months in both WT and Fmr1 null mice. Superimposed on the age-dependent decline in rCPS, we demonstrate a regionally selective elevation in rCPS in Fmr1 null mice. Our results suggest that the process of synaptic pruning during young adulthood may be reflected in decreased rCPS. Our findings support the hypothesis that FMRP is a suppressor of translation in brain in vivo.
Figures

References
-
- Agranoff BW (1981) Learning and memory: biochemical approaches. In: Basic neurochemistry (Seigel GJ, Albers RW, Agranoff BW, Katzman R, eds), pp 801-820. Boston: Little, Brown and Company.
-
- Baubet V, Grange E, Sermet E, Giaume M, Gay N, Bobillier P (1996) Wide-spread increase in brain protein synthesis following acute immobilization stress in adult rat brain. Neurosci Lett 219: 187-190. - PubMed
-
- Bear M, Huber KM, Warren ST (2004) The mGluR theory of fragile X mental retardation. Trends Neurosci 27: 370-377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
