Regulation of membrane localization of Sanpodo by lethal giant larvae and neuralized in asymmetrically dividing cells of Drosophila sensory organs
- PMID: 15901829
- PMCID: PMC1182290
- DOI: 10.1091/mbc.e05-03-0177
Regulation of membrane localization of Sanpodo by lethal giant larvae and neuralized in asymmetrically dividing cells of Drosophila sensory organs
Abstract
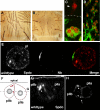
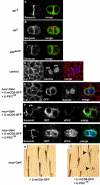
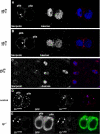
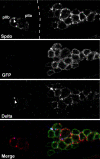
In Drosophila, asymmetric division occurs during proliferation of neural precursors of the central and peripheral nervous system (PNS), where a membrane-associated protein, Numb, is asymmetrically localized during cell division and is segregated to one of the two daughter cells (the pIIb cell) after mitosis. numb has been shown genetically to function as an antagonist of Notch signaling and also as a negative regulator of the membrane localization of Sanpodo, a four-pass transmembrane protein required for Notch signaling during asymmetric cell division in the CNS. Previously, we identified lethal giant larvae (lgl) as a gene required for numb-mediated inhibition of Notch in the adult PNS. In this study we show that Sanpodo is expressed in asymmetrically dividing precursor cells of the PNS and that Sanpodo internalization in the pIIb cell is dependent cytoskeletally associated Lgl. Lgl specifically regulates internalization of Sanpodo, likely through endocytosis, but is not required for the endocytosis Delta, which is a required step in the Notch-mediated cell fate decision during asymmetric cell division. Conversely, the E3 ubiquitin ligase neuralized is required for both Delta endocytosis and the internalization of Sanpodo. This study identifies a hitherto unreported role for Lgl as a regulator of Sanpodo during asymmetric cell division in the adult PNS.
Figures
Similar articles
-
Lethal giant larvae controls the localization of notch-signaling regulators numb, neuralized, and Sanpodo in Drosophila sensory-organ precursor cells.Curr Biol. 2005 May 24;15(10):955-62. doi: 10.1016/j.cub.2005.04.054. Curr Biol. 2005. PMID: 15916953
-
Par3 cooperates with Sanpodo for the assembly of Notch clusters following asymmetric division of Drosophila sensory organ precursor cells.Elife. 2021 Oct 1;10:e66659. doi: 10.7554/eLife.66659. Elife. 2021. PMID: 34596529 Free PMC article.
-
Numb and alpha-Adaptin regulate Sanpodo endocytosis to specify cell fate in Drosophila external sensory organs.EMBO Rep. 2005 Sep;6(9):836-42. doi: 10.1038/sj.embor.7400500. EMBO Rep. 2005. PMID: 16113648 Free PMC article.
-
Spatio-Temporal Regulation of Notch Activation in Asymmetrically Dividing Sensory Organ Precursor Cells in Drosophila melanogaster Epithelium.Cells. 2024 Jun 30;13(13):1133. doi: 10.3390/cells13131133. Cells. 2024. PMID: 38994985 Free PMC article. Review.
-
Lethal giant puzzle of Lgl.Dev Neurosci. 2006;28(1-2):13-24. doi: 10.1159/000090749. Dev Neurosci. 2006. PMID: 16508300 Review.
Cited by
-
Endocytosis by Numb breaks Notch symmetry at cytokinesis.Nat Cell Biol. 2012 Jan 22;14(2):131-9. doi: 10.1038/ncb2419. Nat Cell Biol. 2012. PMID: 22267085
-
Endocytosis and control of Notch signaling.Curr Opin Cell Biol. 2012 Aug;24(4):534-40. doi: 10.1016/j.ceb.2012.06.006. Epub 2012 Jul 18. Curr Opin Cell Biol. 2012. PMID: 22818956 Free PMC article. Review.
-
Endocytosis at the Crossroad of Polarity and Signaling Regulation: Learning from Drosophila melanogaster and Beyond.Int J Mol Sci. 2022 Apr 23;23(9):4684. doi: 10.3390/ijms23094684. Int J Mol Sci. 2022. PMID: 35563080 Free PMC article. Review.
-
Endocytic regulation of Notch signaling.Curr Opin Genet Dev. 2009 Aug;19(4):323-8. doi: 10.1016/j.gde.2009.04.005. Epub 2009 May 15. Curr Opin Genet Dev. 2009. PMID: 19447603 Free PMC article. Review.
-
Numb independently antagonizes Sanpodo membrane targeting and Notch signaling in Drosophila sensory organ precursor cells.Mol Biol Cell. 2010 Mar 1;21(5):802-10. doi: 10.1091/mbc.e09-09-0831. Epub 2010 Jan 6. Mol Biol Cell. 2010. PMID: 20053677 Free PMC article.
References
-
- Arquier, N., Perrin, L., Manfruelli, P., and Semeriva, M. (2001). The Drosophila tumor suppressor gene lethal(2)giant larvae is required for the emission of the Decapentaplegic signal. Development 128, 2209–2220. - PubMed
-
- Barros, C. S., Phelps, C. B., and Brand, A. H. (2003). Drosophila nonmuscle myosin II promotes the asymmetric segregation of cell fate determinants by cortical exclusion rather than active transport. Dev. Cell 5, 829–840. - PubMed
-
- Bellaiche, Y., Radovic, A., Woods, D. F., Hough, C. D., Parmentier, M. L., O'Kane, C. J., Bryant, P. J., and Schweisguth, F. (2001). The Partner of Inscuteable/Discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell 106, 355–366. - PubMed
-
- Berdnik, D., Torok, T., Gonzalez-Gaitan, M., and Knoblich, J. A. (2002). The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell 3, 221–231. - PubMed
-
- Betschinger, J., Eisenhaber, F., and Knoblich, J. A. (2005). Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr. Biol. 15, 276–282. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

