Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia
- PMID: 15902302
- PMCID: PMC1090474
- DOI: 10.1172/JCI24134
Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia
Abstract
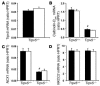
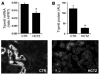
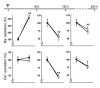
Thiazide diuretics enhance renal Na+ excretion by blocking the Na+-Cl- cotransporter (NCC), and mutations in NCC result in Gitelman syndrome. The mechanisms underlying the accompanying hypocalciuria and hypomagnesemia remain debated. Here, we show that enhanced passive Ca2+ transport in the proximal tubule rather than active Ca2+ transport in distal convolution explains thiazide-induced hypocalciuria. First, micropuncture experiments in mice demonstrated increased reabsorption of Na+ and Ca2+ in the proximal tubule during chronic hydrochlorothiazide (HCTZ) treatment, whereas Ca2+ reabsorption in distal convolution appeared unaffected. Second, HCTZ administration still induced hypocalciuria in transient receptor potential channel subfamily V, member 5-knockout (Trpv5-knockout) mice, in which active distal Ca2+ reabsorption is abolished due to inactivation of the epithelial Ca2+ channel Trpv5. Third, HCTZ upregulated the Na+/H+ exchanger, responsible for the majority of Na+ and, consequently, Ca2+ reabsorption in the proximal tubule, while the expression of proteins involved in active Ca2+ transport was unaltered. Fourth, experiments addressing the time-dependent effect of a single dose of HCTZ showed that the development of hypocalciuria parallels a compensatory increase in Na+ reabsorption secondary to an initial natriuresis. Hypomagnesemia developed during chronic HCTZ administration and in NCC-knockout mice, an animal model of Gitelman syndrome, accompanied by downregulation of the epithelial Mg2+ channel transient receptor potential channel subfamily M, member 6 (Trpm6). Thus, Trpm6 downregulation may represent a general mechanism involved in the pathogenesis of hypomagnesemia accompanying NCC inhibition or inactivation.
Figures



References
-
- Monroy A, Plata C, Hebert SC, Gamba G. Characterization of the thiazide-sensitive Na+-Cl– cotransporter: a new model for ions and diuretics interaction. Am. J. Physiol. Renal Physiol. 2000;279:F161–F169. - PubMed
-
- Hoenderop JGJ, Nilius B, Bindels RJM. Calcium absorption across epithelia. Physiol. Rev. 2005;85:373–422. - PubMed
-
- Costanzo LS, Windhager EE. Calcium and sodium transport by the distal convoluted tubule of the rat. Am. J. Physiol. 1978;235:F492–F506. - PubMed
-
- Ellison DH. Divalent cation transport by the distal nephron: insights from Bartter’s and Gitelman’s syndromes. Am. J. Physiol. Renal Physiol. 2000;279:F616–F625. - PubMed
-
- Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol. Rev. 2000;80:277–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

