Antigen-specific CD4+ T cells drive airway smooth muscle remodeling in experimental asthma
- PMID: 15902312
- PMCID: PMC1088014
- DOI: 10.1172/JCI19711
Antigen-specific CD4+ T cells drive airway smooth muscle remodeling in experimental asthma
Abstract
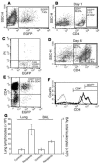
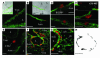
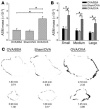
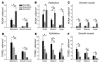
Airway smooth muscle (ASM) growth contributes to the mechanism of airway hyperresponsiveness in asthma. Here we demonstrate that CD4+ T cells, central to chronic airway inflammation, drive ASM remodeling in experimental asthma. Adoptive transfer of CD4+ T cells from sensitized rats induced an increase in proliferation and inhibition of apoptosis of airway myocytes in naive recipients upon repeated antigen challenge, which resulted in an increase in ASM mass. Genetically modified CD4+ T cells expressing enhanced GFP (EGFP) were localized by confocal microscopy in juxtaposition to ASM cells, which suggests that CD4+ T cells may modulate ASM cell function through direct cell-cell interaction in vivo. Coculture of antigen-stimulated CD4+ T cells with cell cycle-arrested ASM cells induced myocyte proliferation, dependent on T cell activation and direct T cell-myocyte contact. Reciprocally, direct cell contact prevented postactivation T cell apoptosis, which suggests receptor-mediated T cell-myocyte crosstalk. Overall, our data demonstrate that activated CD4+ T cells drive ASM remodeling in experimental asthma and suggest that a direct cell-cell interaction participates in CD4+ T cell regulation of myocyte turnover and induction of remodeling.
Figures



References
-
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am. J. Respir. Crit. Care Med. 2000;161:1720–1745. - PubMed
-
- James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am. Rev. Respir. Dis. 1989;139:242–246. - PubMed
-
- Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Pare PD. Functional significance of increased airway smooth muscle in asthma and COPD. J. Appl. Physiol. 1993;74:2771–2781. - PubMed
-
- Robinson DS, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. - PubMed

