Overexpression of MUC13 is associated with intestinal-type gastric cancer
- PMID: 15904467
- PMCID: PMC11160039
- DOI: 10.1111/j.1349-7006.2005.00043.x
Overexpression of MUC13 is associated with intestinal-type gastric cancer
Abstract
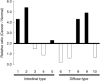
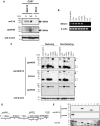
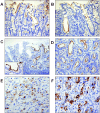
Mucins are secreted or transmembrane glycoproteins that are expressed mainly in the digestive tract. This family of proteins has been the focus of much gastric cancer research as some transmembrane mucins are implicated in tumorigenesis and make attractive targets for cancer diagnosis and therapeutics. Mucins have also been utilized to classify gastric cancer by differentiating between gastric and intestinal phenotypes. Here we show that transmembrane mucin MUC13 is upregulated in gastric cancer. By quantitative real-time reverse transcription-polymerase chain reaction and immunoblot analysis, overexpression of MUC13 was verified in more than half of the samples examined. In immunohistochemical analysis, MUC13 staining was observed in 74 of 114 cases of gastric cancer (64.9%), predominantly in intestinal type (P < 0.001), and in 9 of 10 cases of intestinal metaplasia, precancerous lesions of intestinal-type gastric cancer, but not observed in normal gastric mucosa. Moreover, MUC13 staining patterns characteristic of histological type were identified: staining was on the apical side of tubular glands in intestinal type and on the cytoplasm in diffuse type. These results suggest that MUC13 is a good differentiation marker for gastrointestinal mucosa and that it may have a causal role that correlates with two distinct gastric tumorigenesis pathways.
Figures


References
-
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol 2001; 2: 533–43. - PubMed
-
- Laur’En P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. - PubMed
-
- Nakamura K, Sugano H, Takagi K, Fuchigami A. Histopathological study on early carcinoma of the stomach: some considerations on the ulcer‐cancer by analysis of 144 foci of the superficial spreading carcinomas. Gann 1967; 58: 377–87. - PubMed
-
- Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann 1968; 59: 251–8. - PubMed
-
- Correa P, Chen VW. Gastric cancer. Cancer Surv 1994; 19–20: 55–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

