Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct
- PMID: 15905145
- PMCID: PMC1435688
- DOI: 10.1074/mcp.M500049-MCP200
Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct
Abstract
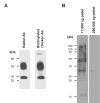
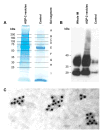
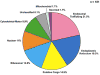
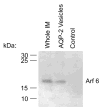
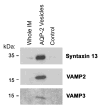
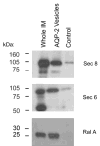
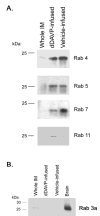
Vasopressin acts on renal collecting duct cells to stimulate translocation of aquaporin-2 (AQP2)-containing membrane vesicles from throughout the cytoplasm to the apical region. The vesicles fuse with the plasma membrane to increase water permeability. To identify the intracellular membrane compartments that contain AQP2, we carried out LC-MS/MS-based proteomic analysis of immunoisolated AQP2-containing intracellular vesicles from rat inner medullary collecting duct. Immunogold electron microscopy and immunoblotting confirmed heavy AQP2 labeling of immunoisolated vesicles. Vesicle proteins were separated by SDS-PAGE followed by in-gel trypsin digestion in consecutive gel slices and identification by LC-MS/MS. Identification of Rab GTPases 4, 5, 18, and 21 (associated with early endosomes); Rab7 (late endosomes); and Rab11 and Rab25 (recycling endosomes) indicate that a substantial fraction of intracellular AQP2 is present in endosomal compartments. In addition, several endosome-associated SNARE proteins were identified including syntaxin-7, syntaxin-12, syntaxin-13, Vti1a, vesicle-associated membrane protein 2, and vesicle-associated membrane protein 3. Rab3 was not found, however, either by mass spectrometry or immunoblotting, suggesting a relative lack of AQP2 in secretory vesicles. Additionally, we identified markers of the trans-Golgi network, components of the exocyst complex, and several motor proteins including myosin 1C, non-muscle myosins IIA and IIB, myosin VI, and myosin IXB. Beyond this, identification of multiple endoplasmic reticulum-resident proteins and ribosomal proteins indicated that a substantial fraction of intracellular AQP2 is present in rough endoplasmic reticulum. These results show that AQP2-containing vesicles are heterogeneous and that intracellular AQP2 resides chiefly in endosomes, trans-Golgi network, and rough endoplasmic reticulum.
Figures




References
-
- van Balkom BW, Graat MP, van Raak M, Hofman E, van der SP, Deen PM. AmJPhysiol Cell Physiol. 2004;286:C372–C379. - PubMed
-
- Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. AmJPhysiol Renal Physiol. 2004;286:F233–F243. - PubMed
-
- Asai T, Kuwahara M, Kurihara H, Sakai T, Terada Y, Marumo F, Sasaki S. Kidney Int. 2003;64:2–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

