Rhythmicity in arterial smooth muscle
- PMID: 15905215
- PMCID: PMC1464779
- DOI: 10.1113/jphysiol.2005.086405
Rhythmicity in arterial smooth muscle
Abstract
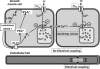
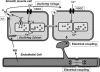
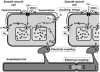
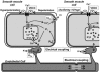
Many arteries and arterioles exhibit rhythmical contractions which are synchronous over considerable distances. This vasomotion is likely to assist in tissue perfusion especially during periods of altered metabolism or perfusion pressure. While the mechanism underlying vascular rhythmicity has been investigated for many years, it has only been recently, with the advent of imaging techniques for visualizing intracellular calcium release, that significant advances have been made. These methods, when combined with mechanical and electrophysiological recordings, have demonstrated that the rhythm depends critically on calcium released from intracellular stores within the smooth muscle cells and on cell coupling via gap junctions to synchronize oscillations in calcium release amongst adjacent cells. While these factors are common to all vessels studied to date, the contribution of voltage-dependent channels and the endothelium varies amongst different vessels. The basic mechanism for rhythmical activity in arteries thus differs from its counterpart in non-vascular smooth muscle, where specific networks of pacemaker cells generate electrical potentials which drive activity within the otherwise quiescent muscle cells.
Figures

References
-
- Bartlett IS, Crane GJ, Neild TO, Segal SS. Electrophysiological basis of arteriolar vasomotion in vivo. J Vasc Res. 2000;37:568–575. - PubMed
-
- Bolton TB, Gordienko DV, Povstyan OV, Harhun MI, Pucovsky V. Smooth muscle cells and interstitial cells of blood vessels. Cell Calcium. 2004;35:643–657. - PubMed
-
- Bonnet S, Belus A, Hyvelin JM, Roux E, Marthan R, Savineau JP. Effect of chronic hypoxia on agonist-induced tone and calcium signaling in rat pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2001;281:L193–L201. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

