Effects of nitroglycerin/L-cysteine on soluble guanylate cyclase: evidence for an activation/inactivation equilibrium controlled by nitric oxide binding and haem oxidation
- PMID: 15907191
- PMCID: PMC1198942
- DOI: 10.1042/BJ20050565
Effects of nitroglycerin/L-cysteine on soluble guanylate cyclase: evidence for an activation/inactivation equilibrium controlled by nitric oxide binding and haem oxidation
Abstract
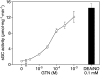
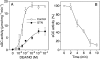
GTN (nitroglycerin; glycerol trinitrate) causes dilation of blood vessels via activation of nitric oxide (NO)-sensitive sGC (soluble guanylate cyclase), a heterodimeric haem protein that catalyses the conversion of GTP into cGMP. Activation of sGC by GTN requires enzymatic or non-enzymatic bioactivation of the nitrate. Based on insufficient NO release and lack of spectroscopic evidence for formation of NO-sGC, the cysteine (Cys)-dependent activation of sGC by GTN was proposed to occur in an NO-independent manner. This extraordinary claim is questioned by the present findings. First, the effect of GTN/Cys was blocked by the NO scavenger oxyhaemoglobin, the superoxide-generating compound flavin mononucleotide and the haem-site sGC inhibitor ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one). Secondly, at equi-effective concentrations, GTN/Cys and the NO donor 2,2-diethyl-1-nitroso-oxyhydrazine released identical amounts of NO. Finally, at sufficiently high rates of NO release, activation of sGC by GTN/Cys was accompanied by a shift of the Soret band from 431 to 399 nm, indicating formation of NO-sGC. In the absence of Cys, GTN caused haem oxidation, apparent as a shift of the Soret band to 392 nm, which was accompanied by inactivation of the NO-stimulated enzyme. These results suggest that the effect of GTN/Cys is the result of an activation/inactivation equilibrium that is controlled by the rate of NO release and haem oxidation.
Figures





Similar articles
-
Effects of nitroglycerin on soluble guanylate cyclase: implications for nitrate tolerance.J Biol Chem. 2002 May 24;277(21):18253-6. doi: 10.1074/jbc.C200170200. Epub 2002 Apr 5. J Biol Chem. 2002. PMID: 11937497
-
Contribution of aldehyde dehydrogenase to mitochondrial bioactivation of nitroglycerin: evidence for the activation of purified soluble guanylate cyclase through direct formation of nitric oxide.Biochem J. 2005 Feb 1;385(Pt 3):769-77. doi: 10.1042/BJ20041354. Biochem J. 2005. PMID: 15377279 Free PMC article.
-
In vitro activation of soluble guanylyl cyclase and nitric oxide release: a comparison of NO donors and NO mimetics.Biochemistry. 2001 Aug 7;40(31):9256-64. doi: 10.1021/bi002885x. Biochemistry. 2001. PMID: 11478893
-
Thiol-Based Redox Modulation of Soluble Guanylyl Cyclase, the Nitric Oxide Receptor.Antioxid Redox Signal. 2017 Jan 20;26(3):137-149. doi: 10.1089/ars.2015.6591. Epub 2016 Apr 1. Antioxid Redox Signal. 2017. PMID: 26906466 Free PMC article. Review.
-
The receptor-like properties of nitric oxide-activated soluble guanylyl cyclase in intact cells.Mol Cell Biochem. 2002 Jan;230(1-2):165-76. Mol Cell Biochem. 2002. PMID: 11952092 Review.
Cited by
-
Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate-Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress.Antioxid Redox Signal. 2015 Oct 10;23(11):899-942. doi: 10.1089/ars.2015.6376. Epub 2015 Sep 24. Antioxid Redox Signal. 2015. PMID: 26261901 Free PMC article. Review.
-
Bioactivation of nitroglycerin by purified mitochondrial and cytosolic aldehyde dehydrogenases.J Biol Chem. 2008 Jun 27;283(26):17873-80. doi: 10.1074/jbc.M801182200. Epub 2008 May 1. J Biol Chem. 2008. PMID: 18450747 Free PMC article.
-
Pharmacological manipulation of cGMP and NO/cGMP in CNS drug discovery.Nitric Oxide. 2019 Jan 1;82:59-74. doi: 10.1016/j.niox.2018.10.006. Epub 2018 Oct 28. Nitric Oxide. 2019. PMID: 30394348 Free PMC article. Review.
-
Glutathione-S-Transferases as Potential Targets for Modulation of Nitric Oxide-Mediated Vasodilation.Biomolecules. 2022 Sep 13;12(9):1292. doi: 10.3390/biom12091292. Biomolecules. 2022. PMID: 36139130 Free PMC article.
-
Partially irreversible inactivation of mitochondrial aldehyde dehydrogenase by nitroglycerin.J Biol Chem. 2008 Nov 7;283(45):30735-44. doi: 10.1074/jbc.M804001200. Epub 2008 Sep 11. J Biol Chem. 2008. PMID: 18786921 Free PMC article.
References
-
- Friebe A., Koesling D. Regulation of nitric oxide-sensitive soluble guanylyl cyclase. Circ. Res. 2003;93:96–105. - PubMed
-
- Krumenacker J. S., Hanafy K. A., Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain. Res. Bull. 2004;15:505–515. - PubMed
-
- Denninger J. W., Marletta M. A. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim. Biophys. Acta. 1999;1411:334–350. - PubMed
-
- Gewaltig M., Kojda G. Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc. Res. 2002;55:250–260. - PubMed
-
- Marsh N., Marsh A. A short history of nitroglycerin and nitric oxide in pharmacology and physiology. Clin. Exp. Pharmacol. Physiol. 2000;27:313–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

