Putative tumor suppressor RASSF1 interactive protein and cell death inducer C19ORF5 is a DNA binding protein
- PMID: 15907802
- PMCID: PMC3225229
- DOI: 10.1016/j.bbrc.2005.05.006
Putative tumor suppressor RASSF1 interactive protein and cell death inducer C19ORF5 is a DNA binding protein
Abstract
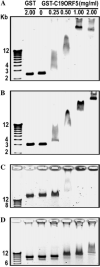
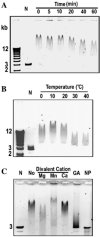
C19ORF5 is a homologue of microtubule-associated protein MAP1B that interacts with natural paclitaxel-like microtubule stabilizer and candidate tumor suppressor RASSF1A. Although normally distributed throughout the cytosol, C19ORF5 specifically associates with microtubules stabilized by paclitaxel or RASSF1A. At sufficiently high concentrations, C19ORF5 causes mitochondrial aggregation and genome destruction (MAGD). The accumulation on hyperstabilized microtubules coupled to MAGD has been proposed to mediate tumor suppression by the taxoid drug family and RASSF1A. Here, we show that the C-terminus of C19ORF5 (C19ORF5C) interacts with mitochondria-associated DNA binding protein, LRPPRC, in liver cells. Like LRPPRC, C19ORF5 also binds DNA with an affinity and specificity sufficient to be of utility in DNA affinity chromatography to purify homogeneous recombinant C19ORF5C from bacterial extracts. Homogeneous C19ORF5 exhibited no intrinsic DNase activity. Deletion mutagenesis indicated that C19ORF5 selectively binds double stranded DNA through its microtubule binding domain. These results suggest C19ORF5 as a DNA binding protein similar to microtubule-associated proteins tau and MAP2.
Figures



References
-
- Liu L, Vo A, Liu G, McKeehan WL. Novel complex integrating mitochondria and the microtubular cytoskeleton with chromosome remodeling and tumor suppressor RASSF1 deduced by in silico homology analysis, interaction cloning in yeast, and colocalization in cultured cells. In Vitro Cell. Dev. Biol. Anim. 2002;38:582–594. - PMC - PubMed
-
- Dallol A, Agathanggelou A, Fenton SL, Ahmed-Choudhury J, Hesson L, Vos MD, Clark GJ, Downward J, Maher ER, Latif F. RASSF1A interacts with microtubule-associated proteins and modulates microtubule dynamics. Cancer Res. 2004;64:4112–4116. - PubMed
-
- Liu L, Vo A, McKeehan WL. Specificity of the methylation-suppressed A isoform of candidate tumor suppressor RASSF1 for microtubule hyperstabilization is determined by cell death inducer C19ORF5. Cancer Res. 2005;65:1830–1838. - PubMed
-
- Song MS, Chang JS, Song SJ, Yang TH, Lee H, Lim DS. The centrosomal protein RABP1 regulates mitotic progression by recruiting RASSF1A to spindle poles. J. Biol. Chem. 2005;280:3920–3927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

