Progestins and progesterone in hormone replacement therapy and the risk of breast cancer
- PMID: 15908197
- PMCID: PMC1974841
- DOI: 10.1016/j.jsbmb.2005.02.014
Progestins and progesterone in hormone replacement therapy and the risk of breast cancer
Abstract
Controlled studies and most observational studies published over the last 5 years suggest that the addition of synthetic progestins to estrogen in hormone replacement therapy (HRT), particularly in continuous-combined regimen, increases the breast cancer (BC) risk compared to estrogen alone. By contrast, a recent study suggests that the addition of natural progesterone in cyclic regimens does not affect BC risk. This finding is consistent with in vivo data suggesting that progesterone does not have a detrimental effect on breast tissue. The increased BC risk found with the addition of synthetic progestins to estrogen could be due to the regimen and/or the kind of progestin used. Continuous-combined regimen inhibits the sloughing of mammary epithelium that occurs after progesterone withdrawal in a cyclic regimen. More importantly, the progestins used (medroxyprogesterone acetate and 19-Nortestosterone-derivatives) are endowed with some non-progesterone-like effects, which can potentiate the proliferative action of estrogens. Particularly relevant seem to be the metabolic and hepatocellular effects (decreased insulin sensitivity, increased levels and activity of insulin-like growth factor-I, and decreased levels of SHBG), which contrast the opposite effects induced by oral estrogen.
Figures



 ) mainly medroxyprogesterone acetate (MPA); (
) mainly medroxyprogesterone acetate (MPA); (
 ) 19-Nortestosterone-derived progestins and (20%) MPA; (*) only 19-Nortestosterone-derived progestins; (▴) mainly 19-Nortestosterone-derived progestins.
) 19-Nortestosterone-derived progestins and (20%) MPA; (*) only 19-Nortestosterone-derived progestins; (▴) mainly 19-Nortestosterone-derived progestins.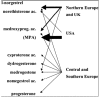
Similar articles
-
Pregnancy, progesterone and progestins in relation to breast cancer risk.J Steroid Biochem Mol Biol. 2005 Dec;97(5):441-50. doi: 10.1016/j.jsbmb.2005.08.015. Epub 2005 Oct 24. J Steroid Biochem Mol Biol. 2005. PMID: 16249080 Review.
-
Hormone replacement therapy and risk of breast cancer: the role of progestins.Acta Obstet Gynecol Scand. 2003 Apr;82(4):335-44. Acta Obstet Gynecol Scand. 2003. Corrected and republished in: Acta Obstet Gynecol Scand. 2003 Jul;82(7):335-44. PMID: 12716318 Corrected and republished. Review.
-
Epidemiologic aspects of exogenous progestagens in relation to their role in pathogenesis of human breast cancer.Acta Endocrinol (Copenh). 1991;125 Suppl 1:13-26. Acta Endocrinol (Copenh). 1991. PMID: 1801500 Review.
-
Progestins and breast cancer.Am J Obstet Gynecol. 2003 May;188(5):1123-31. doi: 10.1067/mob.2003.201. Am J Obstet Gynecol. 2003. PMID: 12748456 Review.
-
Progesterone, progestins and the breast in menopause treatment.Climacteric. 2018 Aug;21(4):326-332. doi: 10.1080/13697137.2018.1476483. Epub 2018 Jun 1. Climacteric. 2018. PMID: 29852797 Review.
Cited by
-
Clarifying hormone terminology.Can Fam Physician. 2007 Jan;53(1):29-30. Can Fam Physician. 2007. PMID: 17872602 Free PMC article. No abstract available.
-
Amphiregulin mediates estrogen, progesterone, and EGFR signaling in the normal rat mammary gland and in hormone-dependent rat mammary cancers.Horm Cancer. 2010 Oct;1(5):229-44. doi: 10.1007/s12672-010-0048-0. Epub 2010 Nov 23. Horm Cancer. 2010. PMID: 21258428 Free PMC article.
-
Risk of breast cancer by type of menopausal hormone therapy: a case-control study among post-menopausal women in France.PLoS One. 2013 Nov 1;8(11):e78016. doi: 10.1371/journal.pone.0078016. eCollection 2013. PLoS One. 2013. PMID: 24223752 Free PMC article.
-
Comparative actions of progesterone, medroxyprogesterone acetate, drospirenone and nestorone on breast cancer cell migration and invasion.BMC Cancer. 2008 Jun 9;8:166. doi: 10.1186/1471-2407-8-166. BMC Cancer. 2008. PMID: 18541028 Free PMC article.
-
Addressing the Missing Links in Cardiovascular Aging.Clin Interv Aging. 2024 May 17;19:873-882. doi: 10.2147/CIA.S457180. eCollection 2024. Clin Interv Aging. 2024. PMID: 38774249 Free PMC article. Review.
References
-
- Clemmesen J. Statistical studies in the aetiology of malignant neoplasms, 3. Acta Pathol Microbiol Scand. 1969;209(S1):S1–S58. - PubMed
-
- Trichopoulos D, MacMahon B, Cole P. Menopause and breast cancer risk. J Natl Cancer Inst. 1972;48(3):605–613. - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351(9114):1451–1467. - PubMed
-
- Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

