Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear
- PMID: 15908345
- PMCID: PMC1111813
- DOI: 10.1128/IAI.73.6.3210-3218.2005
Role of sialic acid and complex carbohydrate biosynthesis in biofilm formation by nontypeable Haemophilus influenzae in the chinchilla middle ear
Abstract
Nontypeable Haemophilus influenzae (NTHI) is an important pathogen in respiratory tract infections, including otitis media (OM). NTHI forms biofilms in vitro as well as in the chinchilla middle ear, suggesting that biofilm formation in vivo might play an important role in the pathogenesis and chronicity of OM. We've previously shown that SiaA, SiaB, and WecA are involved in biofilm production by NTHI in vitro. To investigate whether these gene products were also involved in biofilm production in vivo, NTHI strain 2019 and five isogenic mutants with deletions in genes involved in carbohydrate biosynthesis were inoculated into the middle ears of chinchillas. The wild-type strain formed a large, well-organized, and viable biofilm; however, the wecA, lsgB, siaA, pgm, and siaB mutants were either unable to form biofilms or formed biofilms of markedly reduced mass, organization, and viability. Despite their compromised ability to form a biofilm in vivo, wecA, lsgB, and siaA mutants survived in the chinchilla, inducing culture-positive middle ear effusions, whereas pgm and siaB mutants were extremely sensitive to the bactericidal activity of chinchilla serum and thus did not survive. Lectin analysis indicated that sialic acid was an important component of the NTHI 2019 biofilm produced in vivo. Our data suggested that genes involved in carbohydrate biosynthesis and assembly play an important role in the ability of NTHI to form a biofilm in vivo. Collectively, we found that when modeled in a mammalian host, whereas biofilm formation was not essential for survivability of NTHI in vivo, lipooligosaccharide sialylation was indispensable.
Figures



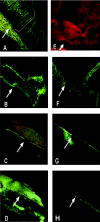
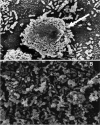

References
-
- Andreoni, J., H. Kayhty, and P. Densen. 1993. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J. Infect. Dis. 168:227-231. - PubMed
-
- Bakaletz, L. O., B. J. Kennedy, L. A. Novotny, G. Duquesne, J. Cohen, and Y. Lobet. 1999. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect. Immun. 67:2746-2762. - PMC - PubMed
-
- Brinkman-Van der Linden, E. C., J. L. Sonnenburg, and A. Varki. 2002. Effects of sialic acid substitutions on recognition by Sambucus nigra agglutinin and Maackia amurensis hemagglutinin. Anal. Biochem. 303:98-104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

