Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis
- PMID: 15908346
- PMCID: PMC1111876
- DOI: 10.1128/IAI.73.6.3219-3227.2005
Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis
Abstract
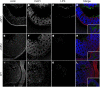
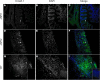
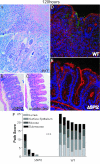
Salmonella species cause a wide range of disease in multiple hosts. Salmonella enterica serovar Typhimurium causes self-limited intestinal disease in humans and systemic typhoid-like illness in susceptible mice. The prevailing dogma in murine S. enterica serovar Typhimurium pathogenesis is that distinct virulence mechanisms-Salmonella pathogenicity islands 1 and 2 (SPI1 and SPI2)-perform distinct roles in pathogenesis, the former being important for invasion and intestinal disease and the latter important for intracellular survival and systemic persistence and disease. Although evidence from bovine infections has suggested that SPI2 has a role in ileal disease, there is no evidence that SPI2 is important for inflammation in a disease that more closely recapitulates human colitis. Using S. enterica serovar Typhimurium strains that lack functional type III secretion systems, we demonstrate that SPI2 is essential for complete virulence in murine infectious enterocolitis. Using a recently characterized murine model (M. Barthel,S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt, Infect. Immun. 71:2839-2858, 2003), we demonstrate that SPI1 mutants are unable to cause intestinal disease 48 h after infection and that SPI2-deficient bacteria also cause significantly attenuated typhlitis. We show that at the peak of inflammation in the cecum, SPI2 mutants induce diminished intercellular adhesion molecule 1 expression and neutrophil recruitment but that wild-type and mutant Salmonella are similarly distributed in the lumen of the infected organ. Finally, we demonstrate that attenuation of intestinal inflammation is accompanied by resolution of typhlitis in the mutant, but not wild-type, infections. Collectively, these results indicate that SPI2 is needed for enterocolitis, as well as for systemic disease.
Figures



Similar articles
-
Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis.Infect Immun. 2005 Nov;73(11):7161-9. doi: 10.1128/IAI.73.11.7161-7169.2005. Infect Immun. 2005. PMID: 16239510 Free PMC article.
-
Comparison of Salmonella enterica serovar Typhimurium colitis in germfree mice and mice pretreated with streptomycin.Infect Immun. 2005 Jun;73(6):3228-41. doi: 10.1128/IAI.73.6.3228-3241.2005. Infect Immun. 2005. PMID: 15908347 Free PMC article.
-
Virulence of broad- and narrow-host-range Salmonella enterica serovars in the streptomycin-pretreated mouse model.Infect Immun. 2006 Jan;74(1):632-44. doi: 10.1128/IAI.74.1.632-644.2006. Infect Immun. 2006. PMID: 16369020 Free PMC article.
-
Salmonella pathogenicity islands in host specificity, host pathogen-interactions and antibiotics resistance of Salmonella enterica.Berl Munch Tierarztl Wochenschr. 2007 Jul-Aug;120(7-8):317-27. Berl Munch Tierarztl Wochenschr. 2007. PMID: 17715824 Review.
-
A mouse model for S. typhimurium-induced enterocolitis.Trends Microbiol. 2005 Oct;13(10):497-503. doi: 10.1016/j.tim.2005.08.008. Trends Microbiol. 2005. PMID: 16140013 Review.
Cited by
-
Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota.PLoS Biol. 2007 Oct;5(10):2177-89. doi: 10.1371/journal.pbio.0050244. PLoS Biol. 2007. PMID: 17760501 Free PMC article.
-
Interleukin-15 and NK1.1+ cells provide innate protection against acute Salmonella enterica serovar Typhimurium infection in the gut and in systemic tissues.Infect Immun. 2009 Jan;77(1):214-22. doi: 10.1128/IAI.01066-08. Epub 2008 Nov 17. Infect Immun. 2009. PMID: 19015253 Free PMC article.
-
IL-17A/F-signaling does not contribute to the initial phase of mucosal inflammation triggered by S. Typhimurium.PLoS One. 2010 Nov 23;5(11):e13804. doi: 10.1371/journal.pone.0013804. PLoS One. 2010. PMID: 21124903 Free PMC article.
-
Salmonella enterica Effectors SifA, SpvB, SseF, SseJ, and SteA Contribute to Type III Secretion System 1-Independent Inflammation in a Streptomycin-Pretreated Mouse Model of Colitis.Infect Immun. 2019 Aug 21;87(9):e00872-18. doi: 10.1128/IAI.00872-18. Print 2019 Sep. Infect Immun. 2019. PMID: 31235639 Free PMC article.
-
The Salmonella Pathogenicity Island (SPI) 1 contributes more than SPI2 to the colonization of the chicken by Salmonella enterica serovar Typhimurium.BMC Microbiol. 2009 Jan 6;9:3. doi: 10.1186/1471-2180-9-3. BMC Microbiol. 2009. PMID: 19126220 Free PMC article.
References
-
- Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839-2858. - PMC - PubMed
-
- Brumell, J. H., C. M. Rosenberger, G. T. Gotto, S. L. Marcus, and B. B. Finlay. 2001. SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell. Microbiol. 3:75-84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

