Comparison of Salmonella enterica serovar Typhimurium colitis in germfree mice and mice pretreated with streptomycin
- PMID: 15908347
- PMCID: PMC1111827
- DOI: 10.1128/IAI.73.6.3228-3241.2005
Comparison of Salmonella enterica serovar Typhimurium colitis in germfree mice and mice pretreated with streptomycin
Abstract
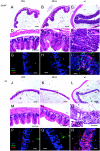
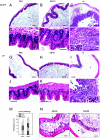
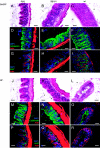
Salmonella enterica subspecies 1 serovar Typhimurium is a common cause of bacterial enterocolitis. Mice are generally protected from Salmonella serovar Typhimurium colonization and enterocolitis by their resident intestinal microflora. This phenomenon is called "colonization resistance" (CR). Two murine Salmonella serovar Typhimurium infection models are based on the neutralization of CR: (i) in specific-pathogen-free mice pretreated with streptomycin (StrSPF mice) antibiotics disrupt the intestinal microflora; and (ii) germfree (GF) mice are raised without any intestinal microflora, but their intestines show distinct physiologic and immunologic characteristics. It has been unclear whether the same pathogenetic mechanisms trigger Salmonella serovar Typhimurium colitis in GF and StrSPF mice. In this study, we compared the two colitis models. In both of the models Salmonella serovar Typhimurium efficiently colonized the large intestine and triggered cecum and colon inflammation starting 8 h postinfection. The type III secretion system encoded in Salmonella pathogenicity island 1 was essential in both disease models. Thus, Salmonella serovar Typhimurium colitis is triggered by similar pathogenetic mechanisms in StrSPF and GF mice. This is remarkable considering the distinct physiological properties of the GF mouse gut. One obvious difference was more pronounced damage and reduced regenerative response of the cecal epithelium in GF mice. Overall, StrSPF mice and GF mice provide similar but not identical models for Salmonella serovar Typhimurium colitis.
Figures




References
-
- Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. - PubMed
-
- Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839-2858. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

