p47 GTPases regulate Toxoplasma gondii survival in activated macrophages
- PMID: 15908352
- PMCID: PMC1111824
- DOI: 10.1128/IAI.73.6.3278-3286.2005
p47 GTPases regulate Toxoplasma gondii survival in activated macrophages
Abstract
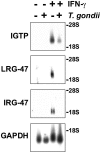
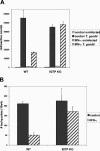
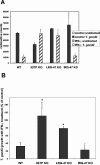
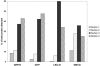
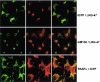
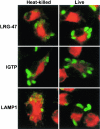
The cytokine gamma interferon (IFN-gamma) is critical for resistance to Toxoplasma gondii. IFN-gamma strongly activates macrophages and nonphagocytic host cells to limit intracellular growth of T. gondii; however, the cellular factors that are required for this effect are largely unknown. We have shown previously that IGTP and LRG-47, members of the IFN-gamma-regulated family of p47 GTPases, are required for resistance to acute T. gondii infections in vivo. In contrast, IRG-47, another member of this family, is not required. In the present work, we addressed whether these GTPases are required for IFN-gamma-induced suppression of T. gondii growth in macrophages in vitro. Bone marrow macrophages that lacked IGTP or LRG-47 displayed greatly attenuated IFN-gamma-induced inhibition of T. gondii growth, while macrophages that lacked IRG-47 displayed normal inhibition. Thus, the ability of the p47 GTPases to limit acute infection in vivo correlated with their ability to suppress intracellular growth in macrophages in vitro. Using confocal microscopy and sucrose density fractionation, we demonstrated that IGTP largely colocalizes with endoplasmic reticulum markers, while LRG-47 was mainly restricted to the Golgi. Although both IGTP and LRG-47 localized to vacuoles containing latex beads, neither protein localized to vacuoles containing live T. gondii. These results suggest that IGTP and LRG-47 are able to regulate host resistance to acute T. gondii infections through their ability to inhibit parasite growth within the macrophage.
Figures


References
-
- Boehm, U., L. Guethlein, T. Klamp, K. Ozbek, A. Schaub, A. Futterer, K. Pfeffer, and J. C. Howard. 1998. Two families of GTPases dominate the cellular response to IFN-γ. J. Immunol. 161:6715-6723. - PubMed
-
- Carlow, D. A., J. Marth, I. Clark-Lewis, and H.-S. Teh. 1995. Isolation of a gene encoding a developmentally regulated T cell-specific protein with a guanidine nucleotide triphosphate binding motif. J. Immunol. 154:1724-1734. - PubMed
-
- Collazo, C. M., G. S. Yap, S. Hieny, P. Caspar, C. G. Feng, G. A. Taylor, and A. Sher. 2002. The function of gamma interferon-inducible GTP-binding protein IGTP in host resistance to Toxoplasma gondii is Stat1 dependent and requires expression in both hematopoietic and nonhematopoietic cellular compartments. Infect. Immun. 70:6933-6939. - PMC - PubMed
-
- Collazo, C. M., G. Yap, G. D. Sempowski, K. D. Lusby, L. Tessarollo, G. F. Vande Woude, G. F., A. Sher, and G. A. Taylor. 2001. Inactivation of LRG-47 and IRG-47 reveals a family of interferon-γ-inducible genes with essential, pathogen-specific roles in resistance to infection. J. Exp. Med. 194:181-187. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

